Industry News
Research, Science & Manufacturer Updates
Therapeutics Articles
Researchers re-engineered the structure of the commonly ussed chemotherapy drug 5-fluorouracil (5FU) into that of a “spherical nucleic acid” — a nanostructure that weaves the drug directly into DNA strands coating tiny spheres, making its cancer cell-killing ability 20,000 more effective, while also reducing its toxicity.
A new study by researchers at Swansea University has revealed a new way to potentially treat certain autoimmune diseases by targeting a protein that helps regulate energy production in immune cells.
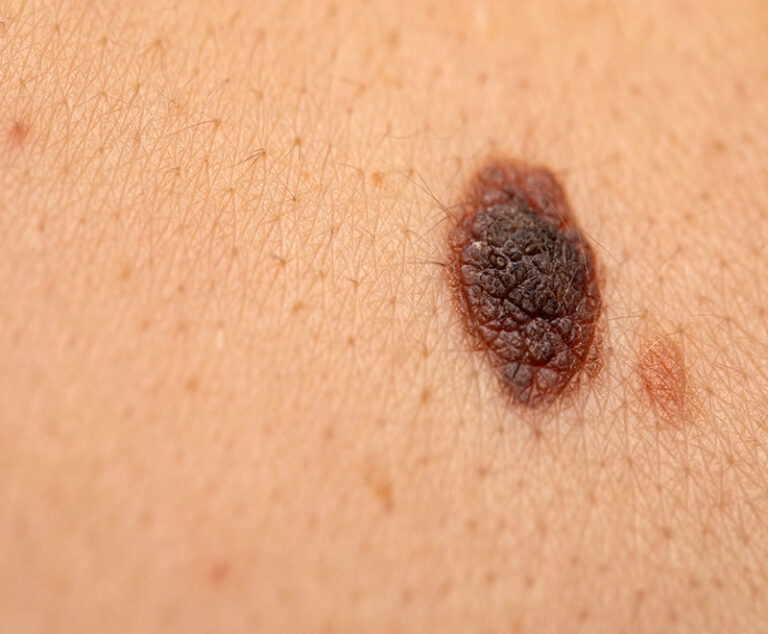
Early data from a Phase II clinical trial show a combination of immunotherapy medications can activate a robust immune response and help overcome treatment resistance in patients with refractory melanoma.
A new study has found that introducing efgartigimod alfa as a treatment option in patients with chronic inflammatory demyelinating polyneuropathy (CIDP) who are currently receiving subcutaneous immune globulin (IG) or intravenous IG would result in substantially increased spending in the treatment of CIPD.
SetPoint System, a neuroimmune modulation device, has been approved by the U.S. Food and Drug Administration to treat adults with moderately to severely active rheumatoid arthritis who have had an inadequate response, loss of response or intolerance to one or more biological or targeted synthetic disease-modifying anti-rheumatic drugs.
The U.S. Food and Drug Administration has granted 510(k) clearance to Takeda’s HyHub and HyHub Duo for patients 17 years of age and older that allow HYQVIA [immune globulin infusion (human), 10% with recombinant human hyaluronidase] to be transferred from vials without using a needle in a home environment or clinical setting.
An investigational, individualized neoantigen therapy, with personalized encoded mRNA, has demonstrated potential to enable patients’ immune systems to target cells that cause cancer.
Scientists at Harvard Medical School have developed a simple nasal spray, made of harmless ingredients, that can protect people against flu, colds and COVID-19 with near-100 percent success, and it costs just $25.
Immune globulin replacement and prophylactic antibiotics are commonly used to prevent infections in patients with secondary hypogammaglobulinemia due to hematological malignancies but have never been directly compared.
A new vaccine currently in development can effectively and affordably lower levels of “bad’ cholesterol in the body, a health problem that affects almost two in five adults in the U.S.
The U.S. Food and Drug Administration has approved two treatments, Casgevy and Lyfgenia, representing the first cell-based gene therapies for the treatment of sickle cell disease in patients 12 years and older.
CSL Behring is discontinuing all sizes of Hizentra vials in the U.S. by the end of September 2024.