Industry News
Research, Science & Manufacturer Updates
The U.S. Food and Drug Administration has approved Apellis Pharmaceuticals’ EMPAVELI (pegcetacoplan) as the first treatment for C3 glomerulopathy (C3G) or primary immune complex membranoproliferative glomerulonephritis (IC-MPGN) in patients 12 years of age and older, to reduce proteinuria.
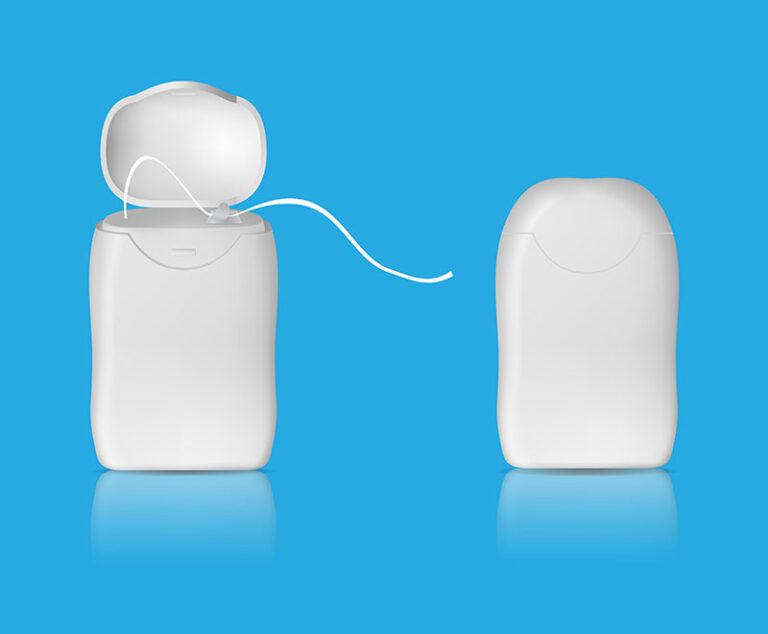
In a proof-of-concept study, researchers at Texas Tech University successfully vaccinated mice against influenza by cleaning their teeth with dental floss coated with inactive flu viruses.
The U.S. Food and Drug Administration has granted 510(k) clearance to Takeda’s HyHub and HyHub Duo for patients 17 years of age and older that allow HYQVIA [immune globulin infusion (human), 10% with recombinant human hyaluronidase] to be transferred from vials without using a needle in a home environment or clinical setting.
Scientists at the University of Florida have developed an experimental mRNA vaccine that, when combined with standard immunotherapy drugs known as immune checkpoint inhibitors, produced a strong antitumor effect in mice, which could lead to a new way of treating cancer without relying solely on surgery, radiation or chemotherapy.
A recent study shows early childhood exposure to aluminum-adsorbed vaccines is not associated with an increased risk for autoimmune, atopic or allergic, or neurodevelopmental disorders.
The U.S. Food and Drug Administration (FDA) has granted full approval for Moderna’s Spikevax (mRNA-1273) COVID vaccine in children 6 months through 11 years who are at an increased risk of the disease.
Results of a Dana-Farber Cancer Institute-initiated Phase I clinical trial for patients with melanoma show that an updated formula and delivery of the NeoVax personalized cancer vaccine called NeoVaxMI is safe, feasible and improves the vaccine-specific immune response compared to previous trials of the platform.
The U.S. Food and Drug Administration has granted accelerated approval for Novartis’ Vanrafia (atrasentan) to reduce proteinuria in adults with primary immunoglobulin A nephropathy (IgAN) at risk of rapid disease progression.
Autism prevalence in the U.S. has increased from one in 36 children to one in 31, according to the Centers for Disease Control and Prevention’s (CDC) latest Autism and Developmental Disabilities Monitoring (ADDM) Network survey.
A recent study found there is significantly higher intravenous immune globulin (IVIG) resistance among children who contracted COVID-19 before developing Kawasaki disease
The University of Toledo will receive $473,632 from the National Institute of Dental and Craniofacial Research to explore how the body’s immune system responds to the condition known as oral thrush.
Researchers at Moffitt Cancer Center have found that tapping into the body’s own immune system and activating a type of immune cell known as B cells could be the key to boosting the effectiveness of tumor-infiltrating lymphocyte, or TIL therapy.