Industry News
Research, Science & Manufacturer Updates
Medicines Articles
Octapharma introduced its newest product, Cutaquig (immune globulin subcutaneous [human] 16.5% solution) at the Immune Deficiency Foundation (IDF) National Conference in June.
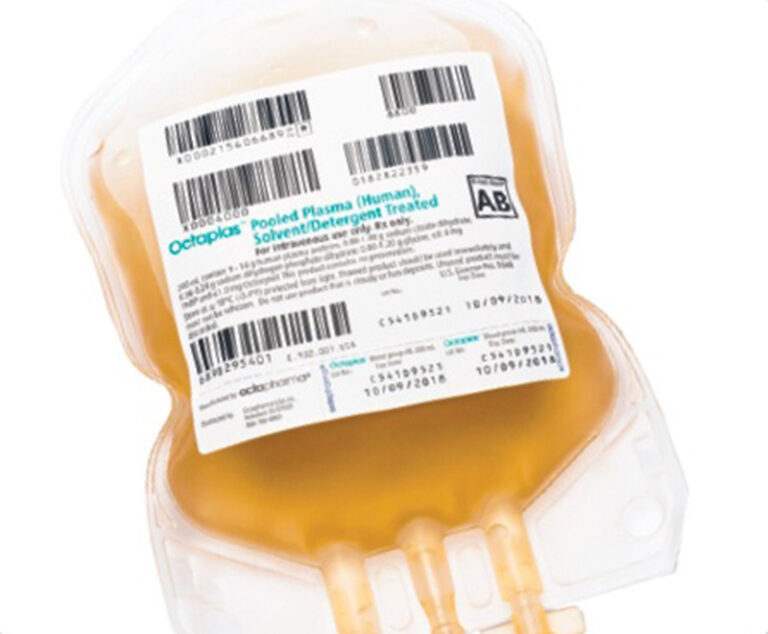
A revised product label for Octapharma USA’s Octaplas (pooled plasma [human] solvent/detergent treated solution for intravenous infusion) to treat critically ill pediatric patients who require replacement of multiple coagulation factors has been approved by the U.S. Food and Drug Administration (FDA).
The National Institutes of Health (NIH) has awarded $945 million to battle addiction and chronic pain.
A trial was conducted to assess the safety and efficacy of hIVIG (in conjunction with standard care) in adults hospitalized with laboratory-confirmed influenza A or B infection.
Researchers at Emory University have described successful use of a novel treatment regimen, dubbed the “Atlanta Protocol,” that involved concomitant use of Hemlibra (emicizumab) prophylaxis and immune tolerance induction (ITI) to treat pediatric patients with severe hemophilia A and inhibitors.
FDA has approved an expanded indication for Dova Pharmaceuticals’ Doptelet (avatrombopag) to treat adults with chronic immune thrombocytopenia.