Industry News
Research, Science & Manufacturer Updates
Disease Indications Articles
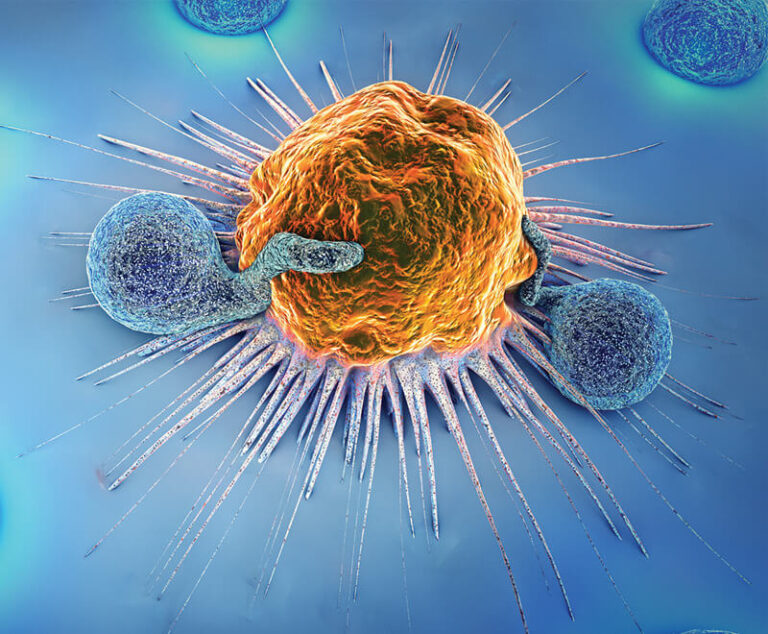
A new study shows that a personalized messenger RNA (mRNA) cancer vaccine plus the checkpoint inhibitor Keytruda (pembrolizumab) reduced the risk of recurrence or death in people with high-risk advanced melanoma.
A large clinical trial of Eli Lilly's experimental Alzheimer's medication, donanemab found the drug slowed declines in patients' ability to think clearly and perform daily tasks by more than a third.
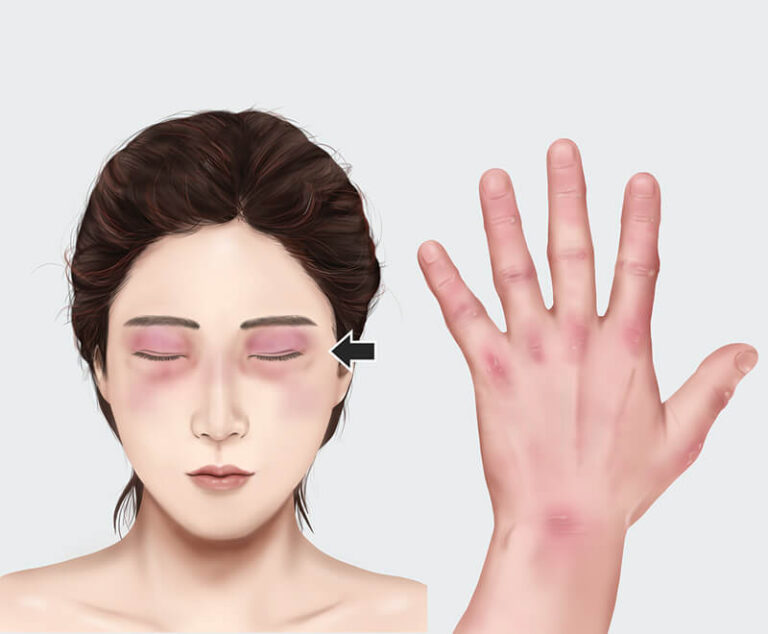
A study evaluating intravenous immune globulin for the treatment of dermatomyositis has found it significantly improved patient outcomes.
Regen BioPharma has filed a provisional patent application covering utilization of dendritic cell technologies to augment efficacy of its patented mRNA cancer immunotherapeutic vaccine.
The World Health Organization has prequalified the first malaria vaccine, RTS,S/AS01 (also known as Mosquirix), manufactured by GSK, bringing it closer to reaching millions more children at risk of malaria.
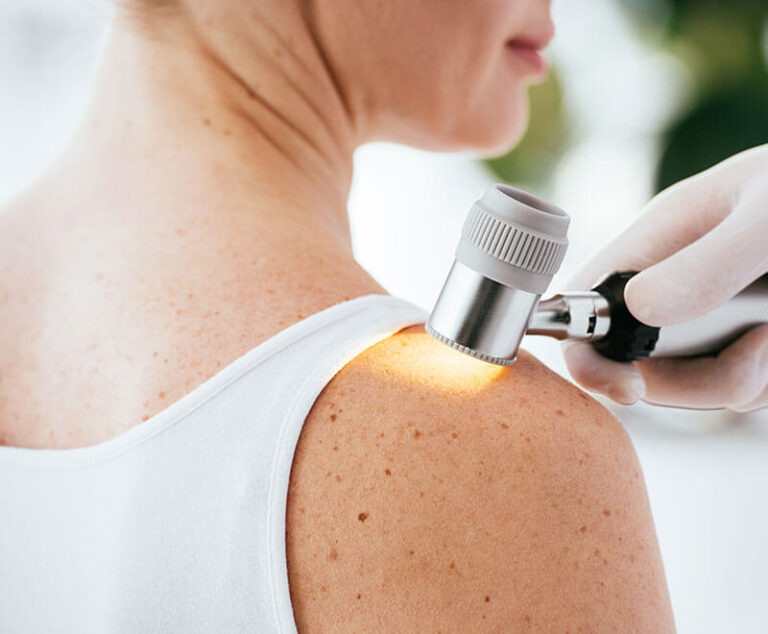
A Phase III clinical trial has found tumor-infiltrating lymphocyte therapy, a new treatment for advanced melanoma, was more effective than ipilimumab, the lading existing therapy.
U.S. researchers have developed a new mRNA vaccine against cancer that delivers the drug directly to the lymphatic system and simulates a strong immune response.
Results of a recent study shows Pfizer's experimental vaccine for respiratory syncytial virus is nearly 86 percent effective in preventing severe illness in older adults.
An experimental vaccine shows promise for protecting mice from the respiratory syncytial virus, the pathogen that most commonly causes the chest infection bronchiolitis in young children.
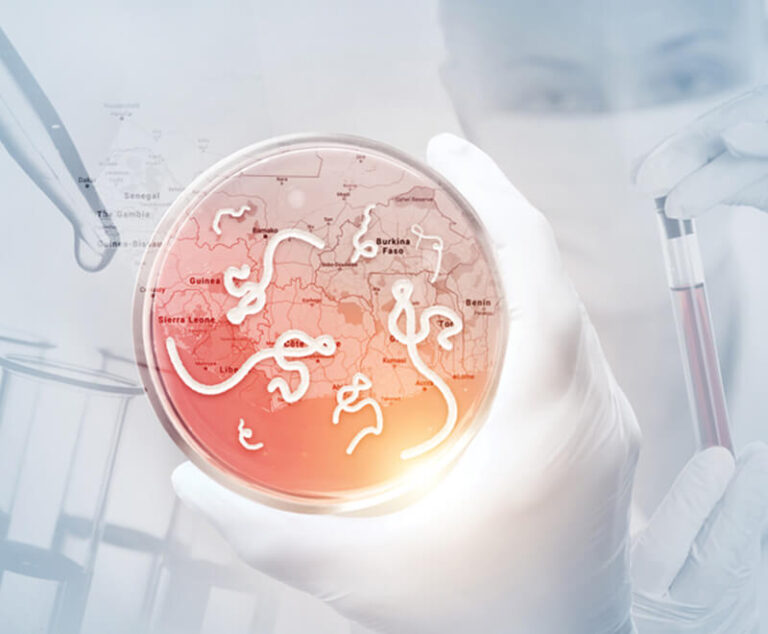
The World Health Organization has published its first guideline for Ebola virus disease therapeutics, with new strong recommendations for the use of two monoclonal antibodies.
In a Phase II trial of participants with hepatic disease associated with congenital alpha1-antitrypsin deficiency, subcutaneous administration of fazirsitran, an investigational RNA interference therapeutic, reduced both serum and hepatic levels of the mutant hepatotoxic AAT protein Z-AAT, with concurrent improvements in enzymatic and histological markers of liver function.
Subcutaneous administration of marstacimab, an investigational human monoclonal antibody targeting tissue factor pathway inhibitor, resulted in a roughly 10-fold lower annualized bleeding rate in 26 hemophilia patients than comparable control individuals in previous clinical trials who were treated on-demand with recombinant factor replacement therapy.