Industry News
Research, Science & Manufacturer Updates
FDA Updates Articles
Genentech’s baloxavir marboxil, an experimental, single-dose flu drug, has been given priority review by the U.S. Food and Drug Administration (FDA).
The U.S. Food and Drug Administration (FDA) has approved Shire’s Cinryze (C1 esterase inhibitor [human]) to prevent angioedema attacks in children 6 years and older with hereditary angioedema.
The U.S. Food and Drug Administration (FDA) has approved Incyte Corp.’s Olumiant (baricitinib) 2 mg once-daily oral medication to treat adults with moderately to severe active rheumatoid arthritis.
The U.S. Food and Drug Administration has approved the first prescription drug made from marijuana.
The U.S. Food and Drug Administration has approved new automated diagnostic tests for lupus and ANCA-associated vasculitis.
The U.S. Food and Drug Administration has approved Genentech’s Rituxan (rituximab) to treat moderate-tosevere phemphigus vulgaris.
The U.S. Food and Drug Administration (FDA) has approved a new, single-dose medication to treat people 12 years and older who have had the flu for no more than 48 hours.
FDA has approved the first generic versions of Mylan’s EpiPen and EpiPen Jr.
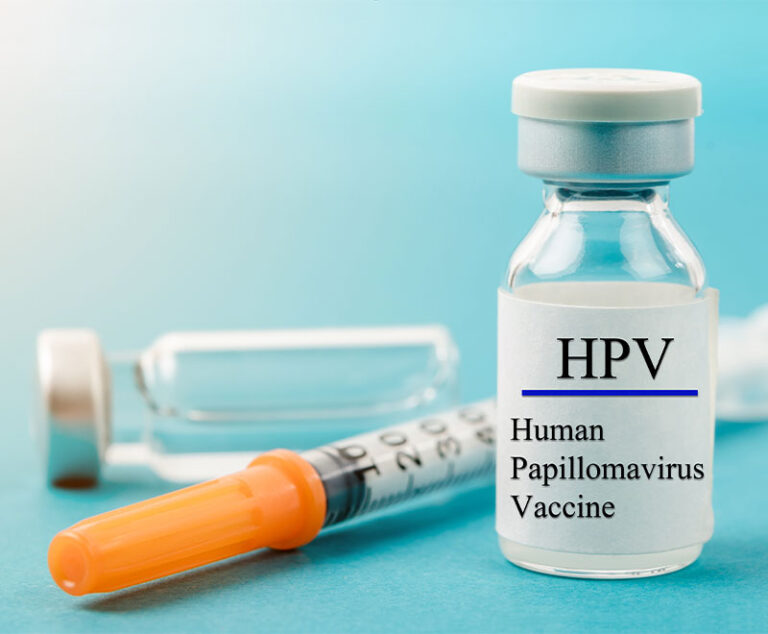
Merck’s Gardasil 9 human papillomavirus (HPV) vaccine has been approved by the U.S. Food and Drug Administration (FDA) for an expanded indication for men and women between the ages of 27 years and 45 years.
The Centers for Disease Control and Prevention's advisory committee has voted 12-2 to recommend FluMist, the nasal spray version of the influenza vaccine, be used during the 2018-19 influenza (flu) season.
The U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee chose the Northern Hemisphere’s 2018-19 influenza (flu) vaccine strains based on the World Health Organization’s recommendations.
The U.S. Food and Drug Administration has approved Erleada (apalutamide) to treat men with prostate cancer that has not yet spread but has a quickly rising PSA level while on treatment with hormone therapy, which causes concern for cancer growth and spread.