Industry News
Research, Science & Manufacturer Updates
FDA Updates Articles
FDA has granted accelerated approval to Keytruda (pembrolizumab) for patients whose cancers have a specific genetic feature (biomarker).
FDA approved Takeda Pharmaceuticals’ second submission for its new plasma manufacturing facility near Covington, Ga.
Meridian Bioscience has received FDA clearance for its new Alethia CMVMolecular Amplification Test (formerly, the Illumigene brand).
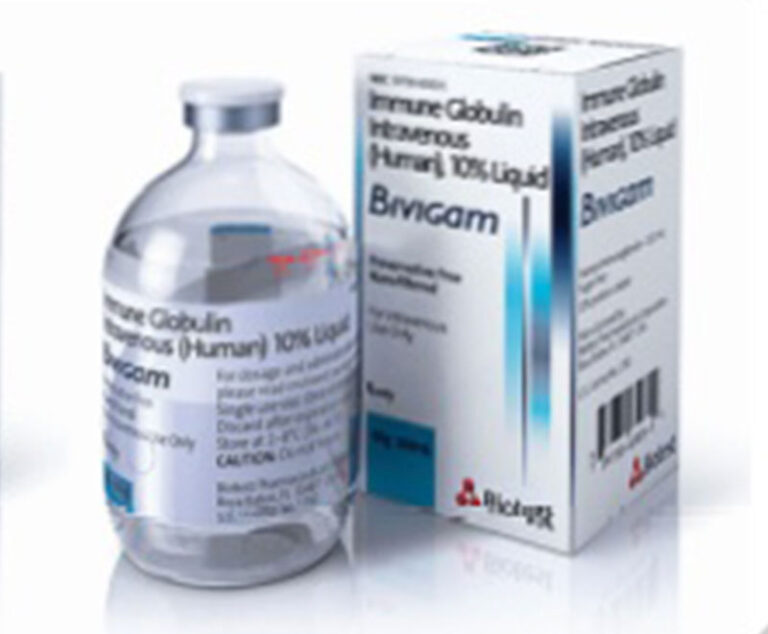
ADMA Biologics has received approval from FDA for its prior approval supplement for Bivigam, allowing the company to use its optimized IVIG manufacturing process and market Bivigam to PI patients in the U.S.
FDA approved Esperoct (antihemophilic factor[recombinant], glycopegylatedexei), an extended half-life factor VIII molecule for replacement therapy in people with hemophilia A.
FDA issuing documents that should lead to smaller clinical trials, faster approvals and quicker launches of nonopioid pain medications.
FDA has approved Cablivi (caplacizumab-yhdp) injection, the first therapy specifically indicated, in combination with plasma exchange and immunosuppressive
therapy, for the treatment of adult patients with acquired thrombotic thrombocytopenic purpura.
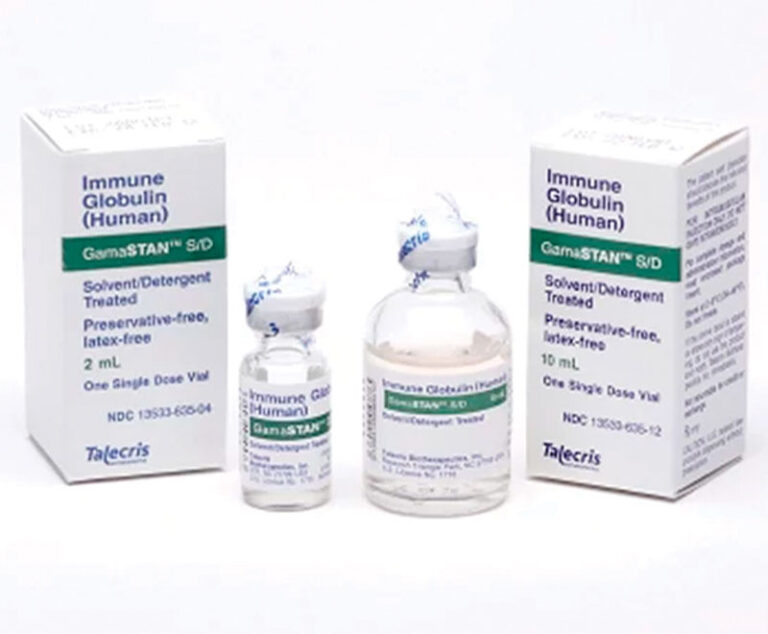
FDA has approved Grifols’ GamaSTAN to treat people exposed to measles and the hepatitis A viruses.
FDA has approved Amgen’s romiplostim to treat pediatric patients ages 1 year and older with immune thrombocytopenia for a minimum of six months and who have had an insufficient response to corticosteroids, immune globulin or splenectomy.
FDA has approved Xospata (gilteritinib) to treat adult patients with relapsed or refractory acute myeloid leukemia with a certain genetic mutation.
FDA granted Sanofi Pasteur’s Adacel Tdap absorbed vaccine expanded indication to include repeat vaccinations for tetanus, diphtheria and pertussis.
The U.S. Food and Drug Administration (FDA) has approved Bayer’s Jivi (BAY94-9027) as a preventive treatment for bleeding in hemophilia A.