Industry News
Research, Science & Manufacturer Updates
Disease Indications Articles
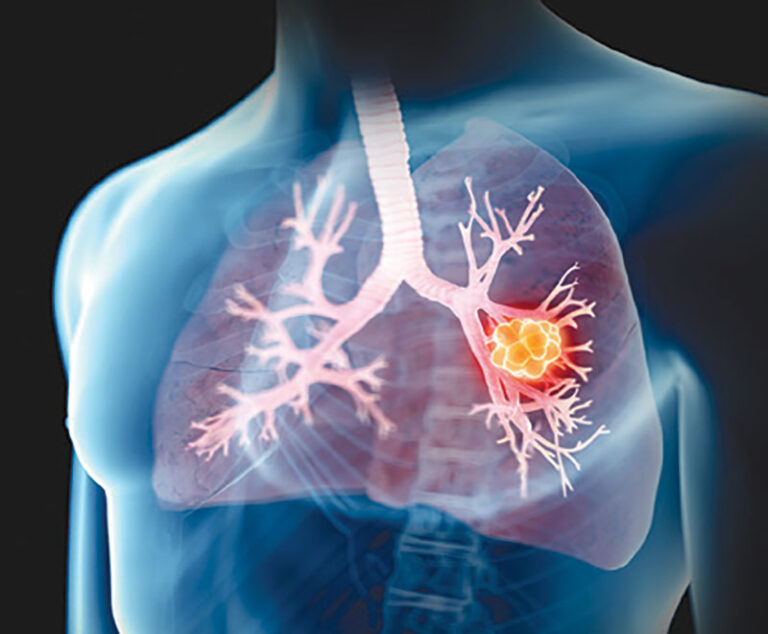
The U.S. Food and Drug Administration has approved Bristol Myers Squibb’s repotrectinib (Augtryo) for the treatment of adult patients with locally advanced or metastatic ROS1-positive non-small cell lung cancer.
The U.S. Food and Drug Administration has approved Tyenne (tocilizumab-aazg), a biosimilar referencing tocilizumab (Actemra; Genentech), to treat multiple autoimmune diseases, including rheumatoid arthritis and juvenile idiopathic arthritis.
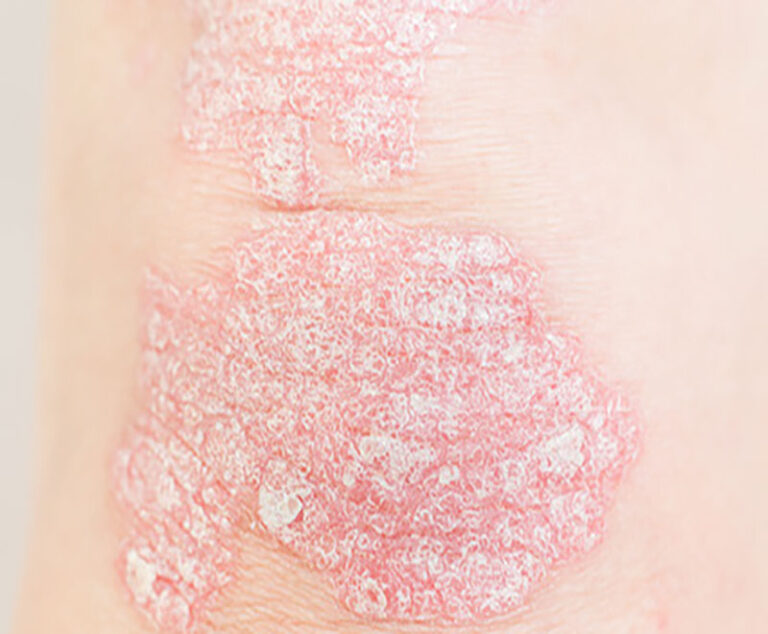
The U.S. Food and Drug Admini-stration has approved Selarsdi (ustekinumab-aekn) injection for subcutaneous use, as a biosimilar to Stelara, for the treatment of moderate to severe plaque psoriasis and for active psoriatic arthritis in adults and pediatric patients 6 years and older.
Researchers at the University of California, San Francisco, have identified a specific pattern of autoantibodies in the blood that precedes the clinical onset of multiple sclerosis.
The U.S. Food and Drug Administration (FDA) has granted orphan drug exclusivity for Octapharma’s wilate, von Willebrand factor/coagulation factor VIII complex (human) lyophilized powder for solution for intravenous injection, for routine prophylaxis to reduce the frequency of bleeding episodes in adults and children 6 years of age and older with von Willebrand disease (VWD).
The Phase IIIb study of emicizumab shows promise for reducing risk of spontaneous and traumatic bleeds in infants with hemophilia A.
Researchers have discovered a rare type of immune cell that may predict how likely some patients with skin cancer will respond to immunotherapy treatment.
Researchers at City of Hope have discovered that a type of immune cell in the human body known to be important for allergy and other immune responses can also attack cancer.
A new vaccine may help speed up the process of making antibodies against SARS-CoV-2 by using preexisting immunity to a separate virus (the influenza virus).
A new cancer vaccine designed to detect and fight cancer cells without traditional cancer treatment is entering Phase III clinical trials.
A recent study has found that a commercially available plasma p-tau217 (phosphorylated tau 217) immunoassay accurately identified biological Alzheimer’s disease (AD).
In a study, researchers found that fSCIG 10% was more effective in preventing CIDP relapse than placebo, supporting its potential use as maintenance CIDP treatment.