Industry News
Research, Science & Manufacturer Updates
CMS has launched the Artificial Intelligence Health Outcomes Challenge, a three-stage competition to accelerate artificial intelligence solutions that will potentially be used by CMS’s Innovation Center.
The FDA is making efforts as part of its Youth Tobacco Prevention Plan to ensure no tobacco products are marketed to, sold to or used by kids.
Two studies suggest low serum albumin levels in patients with sepsis is associated with mortality.
CMS has finalized its rule to give Medicare Advantage plans a 2.53 percent pay raise in 2020, up from its original plan to raise pay 1.59 percent.
CMS announced the Part D Payment Modernization Model to share in savings generated by reducing costs in the program's catastrophic phase.
In 2018, HHS awarded $2.34 billion in Ryan White HIV/AIDS Program grants to cities, counties, states and local community based organizations.
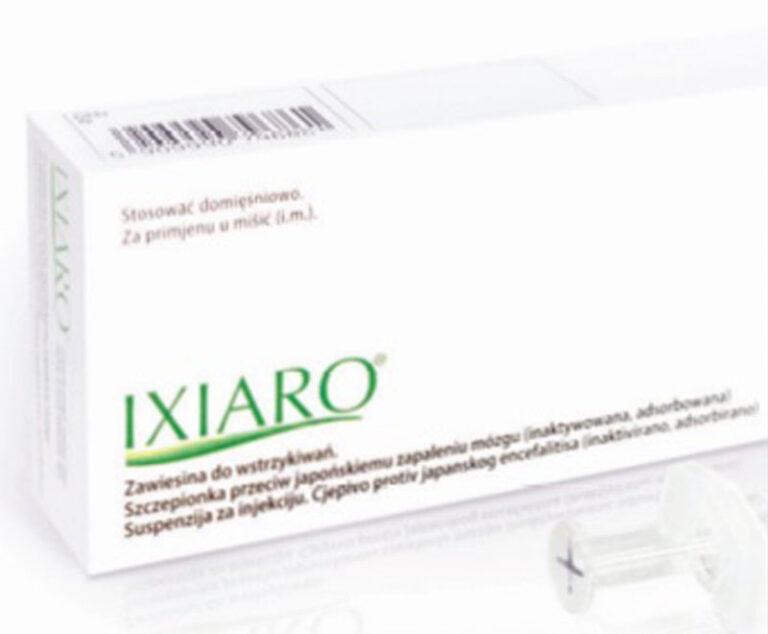
Valneva USA has received FDA approval of an accelerated dosing regimen for IXIARO (Japanese encephalitis vaccine, inactivated, adsorbed).
Pfizer’s Nivestym (filgrastim-aafi) has been approved by FDA for all eligible indications of the reference product.
A new study conducted by investigators at the Center for Clinical Epidemiology and Population Health at the Marshfield Clinic Research Institute in Wisconsin has found the flu vaccine does not cause miscarriages in pregnant women.
FDA has granted accelerated approval to Keytruda (pembrolizumab) for patients whose cancers have a specific genetic feature (biomarker).
FDA approved Takeda Pharmaceuticals’ second submission for its new plasma manufacturing facility near Covington, Ga.