Industry News
Research, Science & Manufacturer Updates
Investigators conducted a retrospective review of 38 cases treated at Taipei Veterans General Hospital between January 2007 and December 2018 to determine whether more aggressive albumin supplementation can benefit major burn patients with persistent hypoalbuminemia.
In pharmacokinetic studies, a recombinant fusion protein genetically linking human coagulation factor IX with human albumin (rIX-FP) (IDELVION, CSL Behring) has been shown to have an approximately five-fold longer half-life compared with standard recombinant factor IX products.
A recent experiment found offering healthcare providers financial incentives and creating competition by informing clinics how their performance ranked relative to others were effective in increasing influenza (flu) vaccine rates among patients.
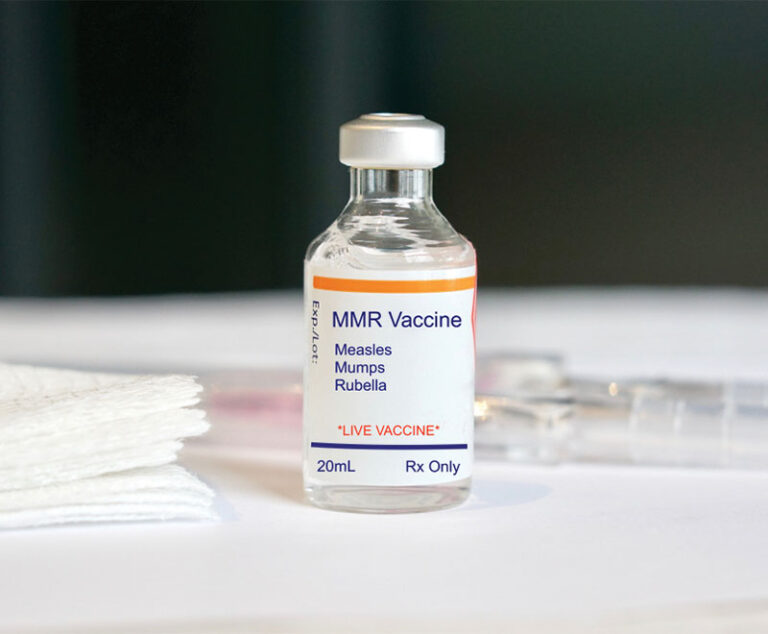
Two studies were conducted to determine whether measles infection causes long-term damage to immune memory.
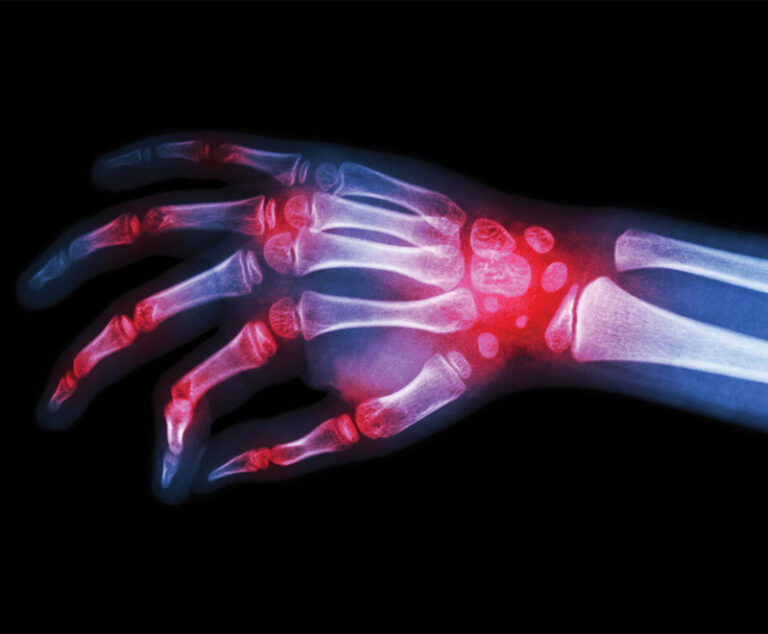
Amgen’s biosimilar to Johnson & Johnson’s rheumatoid arthritis drug, Remicade, has been approved by the U.S. Food and Drug Administration.
Following a review by the Independent Data Monitoring Committee (IDMC) of preliminary results from the Phase II portion of an ongoing Phase II/III trial evaluating Kevzara (sarilumab), the trial was amended so only critical patients continue to be enrolled to receive Kevzara 400 mg or placebo.
Octapharma USA is supporting a new investigator-initiated clinical trial led by George Sakoulas, MD, of Sharp Memorial Hospital in San Diego, Calif., focused on treating the most critical coronavirus patients who are experiencing respiratory failure who become ventilator dependent.
Novartis has initiated a Phase III clinical trial to examine the efficacy of utilizing canakinumab (Ilaris), an interleukin (IL)-1β blocker, to treat a type of severe immune overreaction called cytokine release syndrome (CRS) in people with COVID-19 pneumonia.
Inato, a marketplace that helps biopharmaceutical companies increase the pool of available patients engaged in clinical trials, has unveiled its anticovid platform, a comprehensive, central repository for all existing clinical trials for SARS-CoV 2 (the virus that causes COVID-19).
Epidemiological data suggests populations with the highest measles-mumps-rubella (MMR) vaccination rates often have the fewest deaths from COVID-19.
The Centers for Medicare and Medicaid Services (CMS) has broadened access to Medicare telehealth services so beneficiaries can receive a wider range of services from their doctors without having to travel to a healthcare facility.
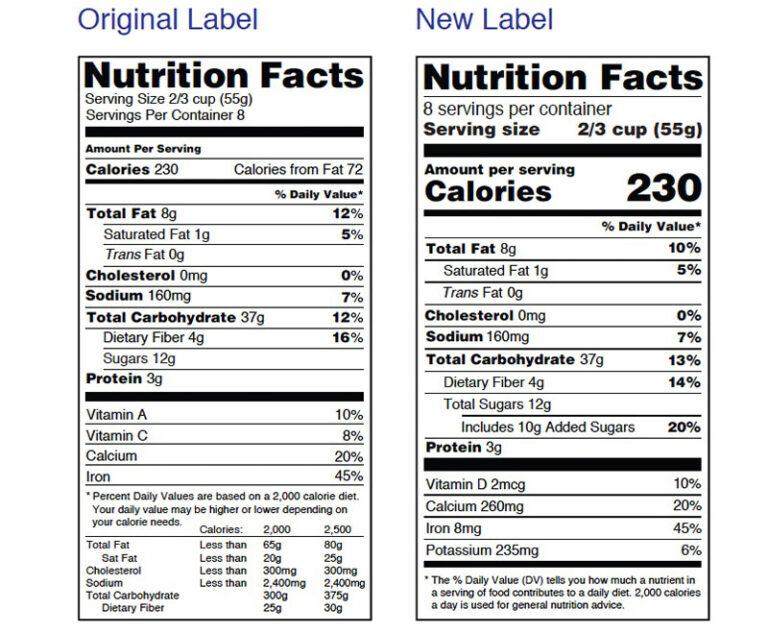
The U.S. Food and Drug Administration (FDA) has launched a campaign to help consumers use the new Nutrition Facts label that appears on packaged foods to maintain healthy dietary practices.