Industry News
Research, Science & Manufacturer Updates
FDA Updates Articles
The U.S. Food and Drug Administration (FDA) issued a final rule to require new health warnings on cigarette packages and in cigarette advertisements, which feature textual statements with photo-realistic color images depicting some of the lesser-known, but serious health risks of cigarette smoking, including impact to fetal growth, cardiac disease, diabetes and more.
The U.S. Food and Drug Administration (FDA) has approved inebilizumab-cdon (Uplizna) to treat neuromyelitis optica spectrum disorder (NMOSD) in adult patients with the anti-AQP4 antibody.
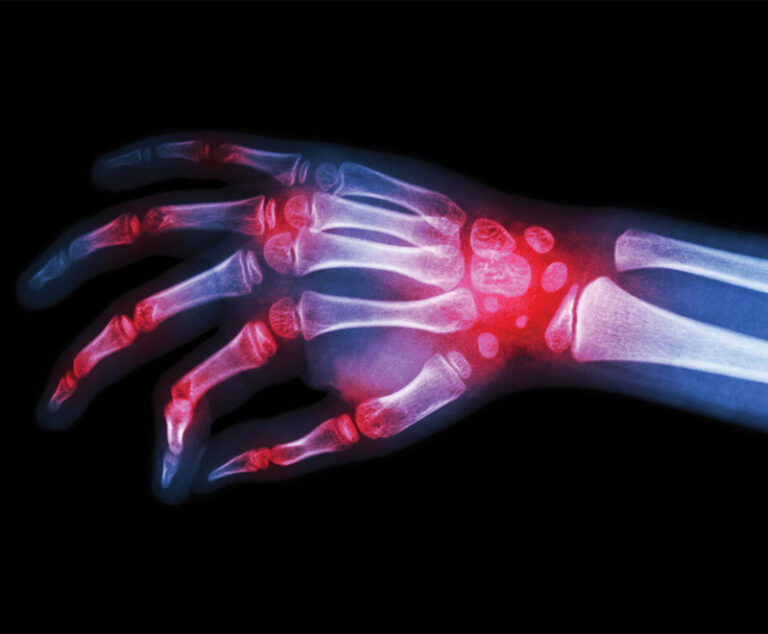
Amgen’s biosimilar to Johnson & Johnson’s rheumatoid arthritis drug, Remicade, has been approved by the U.S. Food and Drug Administration.
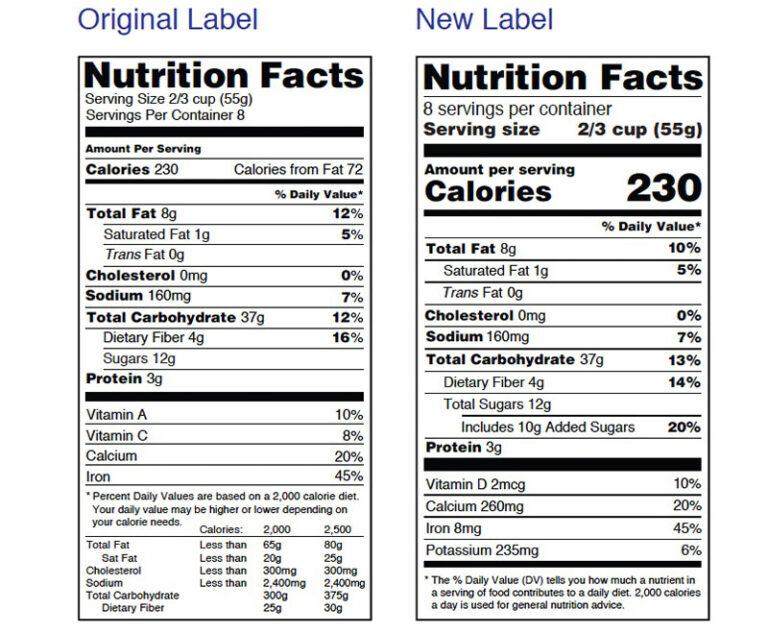
The U.S. Food and Drug Administration (FDA) has launched a campaign to help consumers use the new Nutrition Facts label that appears on packaged foods to maintain healthy dietary practices.
The U.S. Food and Drug Administration (FDA) has approved WILATE for treatment of adults and adolescents with hemophilia A for routine prophylaxis.
The U.S. Food and Drug Administration (FDA) has approved Ziextenzo (pegfilgrastim-bmez), the 24th biosimilar approval in the U.S.
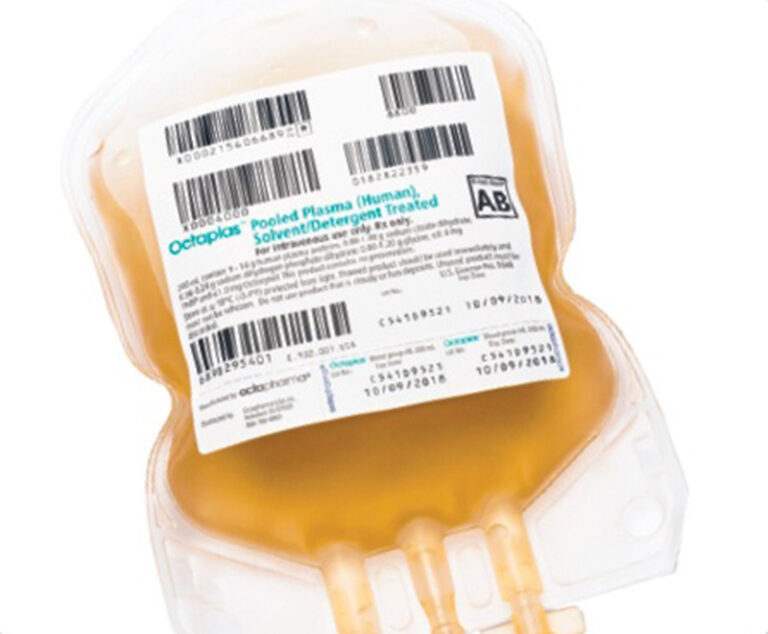
A revised product label for Octapharma USA’s Octaplas (pooled plasma [human] solvent/detergent treated solution for intravenous infusion) to treat critically ill pediatric patients who require replacement of multiple coagulation factors has been approved by the U.S. Food and Drug Administration (FDA).
The U.S. Food and Drug Administration (FDA) released guidelines on the studies companies need to conduct to show their biosimilar is interchangeable with a biologic.
FDA has approved an expanded indication for Dova Pharmaceuticals’ Doptelet (avatrombopag) to treat adults with chronic immune thrombocytopenia.
Sanofi and Merck’s Vaxelis has been approved by the U.S. Food and Drug Administration.
Valneva USA has received FDA approval of an accelerated dosing regimen for IXIARO (Japanese encephalitis vaccine, inactivated, adsorbed).
Pfizer’s Nivestym (filgrastim-aafi) has been approved by FDA for all eligible indications of the reference product.