Industry News
Research, Science & Manufacturer Updates
A study that investigated the effects of tattoo ink on the body has found that the ink travels through the lymphatic system and kills immune cells, as well as alters the effects of some vaccines.
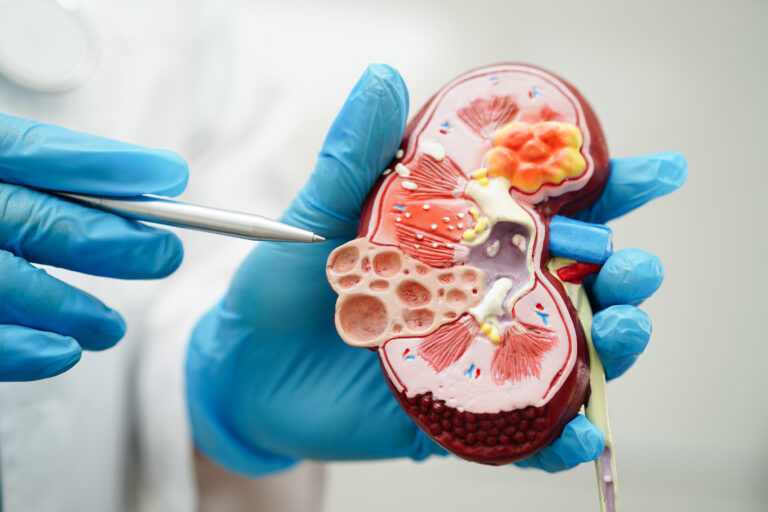
The U.S. Food and Drug Administration (FDA) has granted accelerated approval of VOYXACT (sibeprenlimab-szsi) for the reduction of proteinuria in adults with primary immunoglobulin A nephropathy (IgAN) at risk for disease progression.
Researchers at Ben-Gurion University of the Negev in Israel have discovered a way to destroy harmful “senescent” cells that accumulate with age and increase the kind of tissue damage and inflammation that slowly causes life to end.
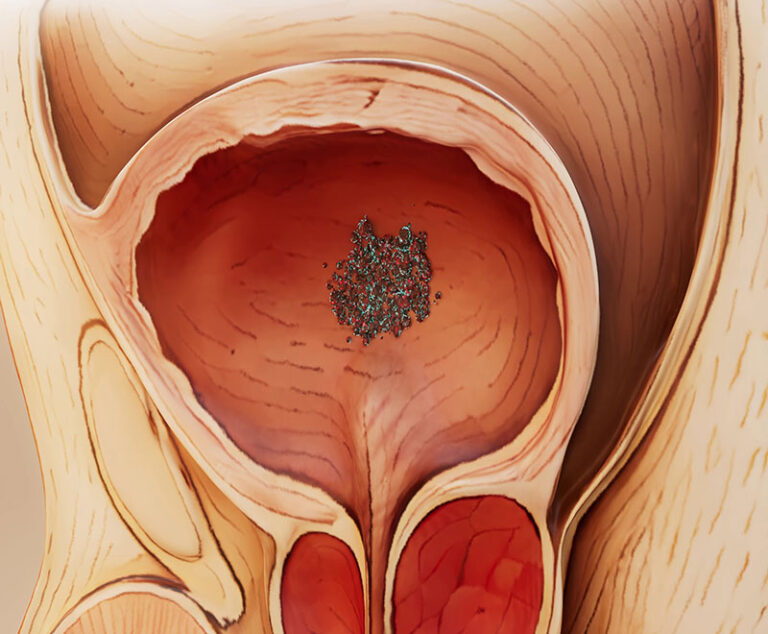
The U.S. Food and Drug Administration has approved KEYTRUDA and KEYTRUDA QLEX in combination with Padcev, as neoadjuvant treatment and then continued after cystectomy as adjuvant treatment, for the treatment of adult patients with muscle-invasive bladder cancer who are ineligible for cisplatin-based chemotherapy.
Researchers re-engineered the structure of the commonly ussed chemotherapy drug 5-fluorouracil (5FU) into that of a “spherical nucleic acid” — a nanostructure that weaves the drug directly into DNA strands coating tiny spheres, making its cancer cell-killing ability 20,000 more effective, while also reducing its toxicity.
A new study by researchers at Swansea University has revealed a new way to potentially treat certain autoimmune diseases by targeting a protein that helps regulate energy production in immune cells.
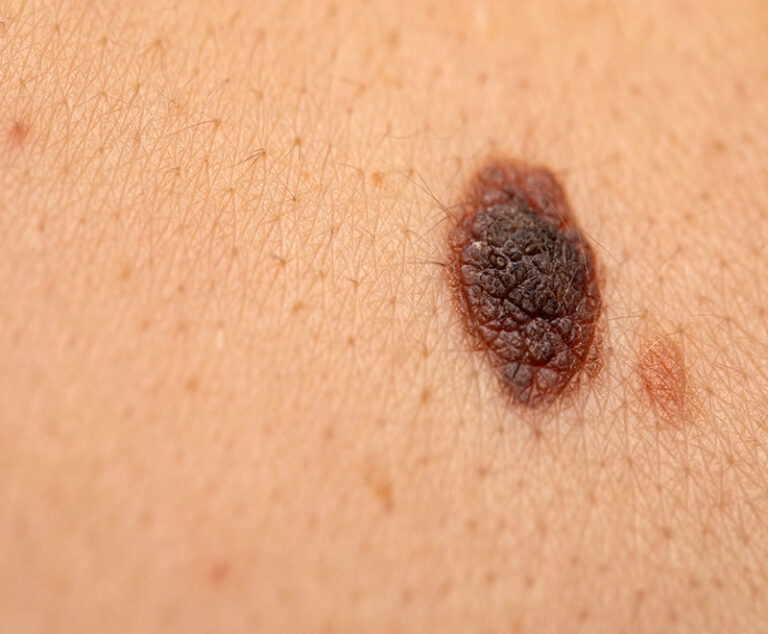
Early data from a Phase II clinical trial show a combination of immunotherapy medications can activate a robust immune response and help overcome treatment resistance in patients with refractory melanoma.
A new study has found that introducing efgartigimod alfa as a treatment option in patients with chronic inflammatory demyelinating polyneuropathy (CIDP) who are currently receiving subcutaneous immune globulin (IG) or intravenous IG would result in substantially increased spending in the treatment of CIPD.
A study has found that for patients with certain types of advanced lung or skin cancer, administration of a SARS-CoV-2 mRNA vaccine within 100 days of starting immune checkpoint inhibitors is associated with increased overall survival.
A retrospective cohort study has found respiratory syncytial virus infections are associated with substantial morbidity among patients with systemic autoimmune rheumatic diseases, with more than half of infected individuals requiring hospitalization.
A new study has found people have an increased risk of major cardiovascular events following viral infections such as SARS-CoV-2, influenza, HIV, hepatitis C and varicella zoster virus.
A recent study shows the use of hormone therapy in postmenopausal women was associated with a significantly higher incidence and risk of autoimmune diseases.