Industry News
Research, Science & Manufacturer Updates
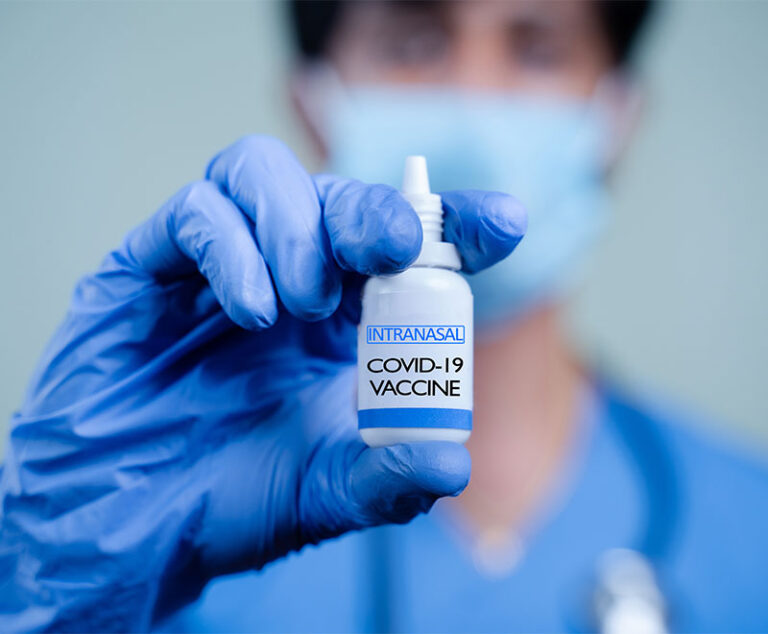
A Phase I randomized, double-blind, placebo-controlled dose-escalation study in healthy adults, called CDX-CoV-001, has found Codagenix’s intranasal COVID-19 vaccine candidate, CoviLiv, showed the vaccine had a high seroresponse rate and induced mucosal immunity in volunteers not previously vaccinated or infected.
Results from Moderna’s Phase I/II clinical trial that evaluated the safety and immunogenicity of its influenza-COVID-19 vaccine, mRNA 1083, showed immunogenicity against all four influenza strains compared to a standard dose of the influenza vaccine, Fluarix, in adults 50 to 64 years of age and against an enhanced influenza vaccine, Fluzone HD, in adults 65 to 79 years of age.
Investigators at the Icahn School of Medicine at Mount Sinai have designed an RNA-based strategy to activate dendritic cells, which play a key role in immune response, that eradicated tumors and prevented their recurrence in mouse models of melanoma.
The combination of intravenous immune globulin (IVIG) and corticosteroids is a more efficient and rapid treatment for relapsed immune thrombocytopenic purpura (ITP) in adults compared with the use of either therapy alone.
A study has found that early albumin administration in septic shock patients with ARDS was independently associated with a reduction in 28-day mortality.
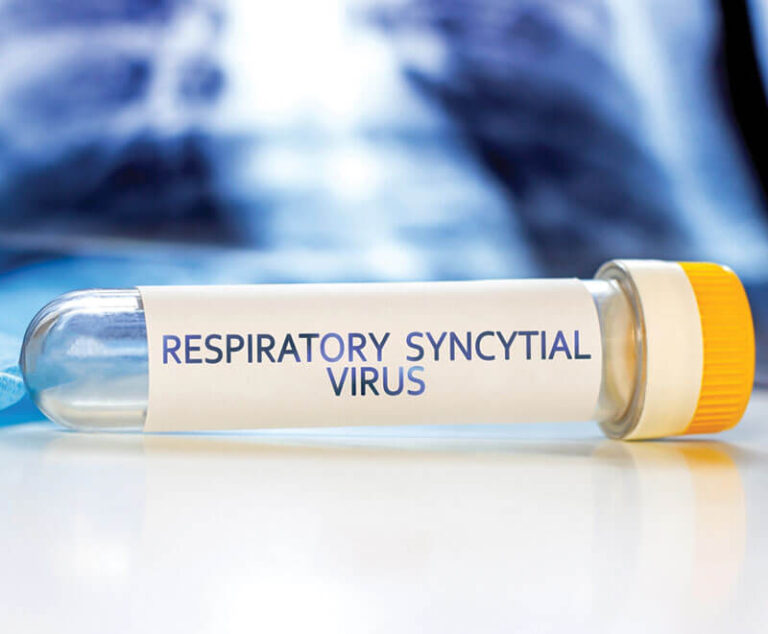
The U.S. Food and Drug Administration (FDA) has approved nirsevimab to protect newborns from respiratory syncytial virus (RSV).
The U.S. Food and Drug Administration has approved Zurzuvae (zuranolone), the first oral medication indicated to treat postpartum depression (PPD) in adults.
A study led by scientists at Oregon Health & Science University has found that a type of monoclonal antibody already tested in certain forms of cancer may be a promising treatment in stopping the progression of amyotrophic lateral sclerosis (ALS).
The U.S. Department of Health and Human Services has founded the Office of Long COVID Research and Practice to lead the Long COVID response and coordination across the federal government. In addition, the National Institutes of Health launched Long COVID clinical trials through the RECOVER Initiative.
The U.S. Department of Health and Human services has awarded more than $147 million to 49 recipients to advance the Ending the HIV Epidemic in the U.S. initiative, which is part of the ongoing efforts to reduce the number of new infections in the United States by at least 90 percent by 2030.
Through the Health Resources and Services Administration, the U.S. Department of Health and Human Services has announced the availability of approximately $25 million to expand primary healthcare, including mental health services, in schools.
The Guiding an Improved Dementia Experience (GUIDE) Model, which aims to improve the quality of life for people living with dementia, reduce strain on unpaid caregivers, and help people remain in their homes and communities through a package of care coordination and management, caregiving education and support, and respite services has been established by the U.S. Department of Health and Human Services.