Industry News
Research, Science & Manufacturer Updates
The Department of Health and Human Services (HHS) announced more than $413 million in Provider Relief Fund (PRF) payments to more than 3,600 providers across the country.
New federal protections shield millions of consumers from surprise medical bills, which are unexpected bills from out-of-network providers, out-of-network facilities or out-of-network air ambulance providers.
An investigational antisense oligo-nucleotide (donidalorsen) that acts by degrading plasma prekallikrein messenger RNA was evaluated in patients with hereditary angioedema (HAE) and C1 inhibitor deficiency to assess whether this agent can reduce the frequency of angioedema attacks and the burden of disease.
A new systematic review and meta-analysis conducted by U.S. and Nepalese collaborators supports the use of IVIG with glucocorticoids compared to IVIG alone.
Researchers at the Texas Children’s Hospital Center for Vaccine Development at Baylor College of Medicine are developing a COVID-19 vaccine using a conventional method that will make the production and distribution cheaper and more accessible.
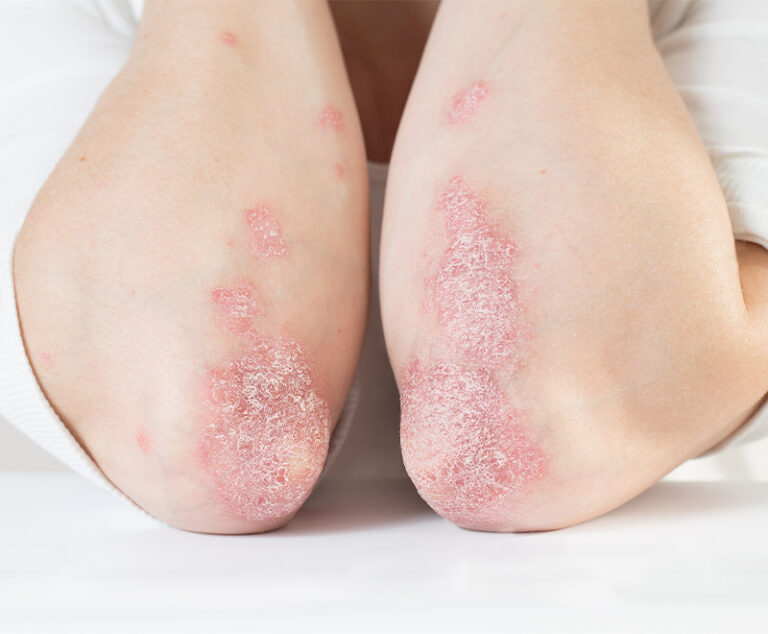
AbbVie’s Rinvoq and Pfizer’s Cibinqo have been approved by the U.S. Food and Drug Administration (FDA) to treat moderate-to-severe atopic dermatitis.
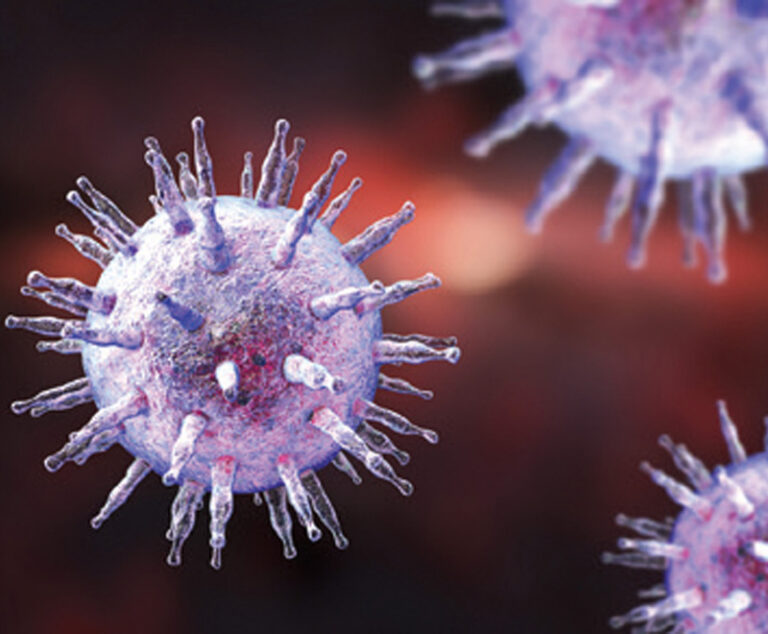
A new study shows the Epstein-Barr virus (EBV) is essential for the development of multiple sclerosis (MS).
The U.S. Food and Drug Administration (FDA) has approved Vyvgart (efgartigi-mod) to treat generalized myasthenia gravis (gMG) in adults who test positive for the anti-acetylcholine receptor (AChR) antibody.
The COVID-19 pandemic highlighted gaps the healthcare system. Two improvements to Medicare in 2022 seek to give people greater access to telehealth and other care delivery options.
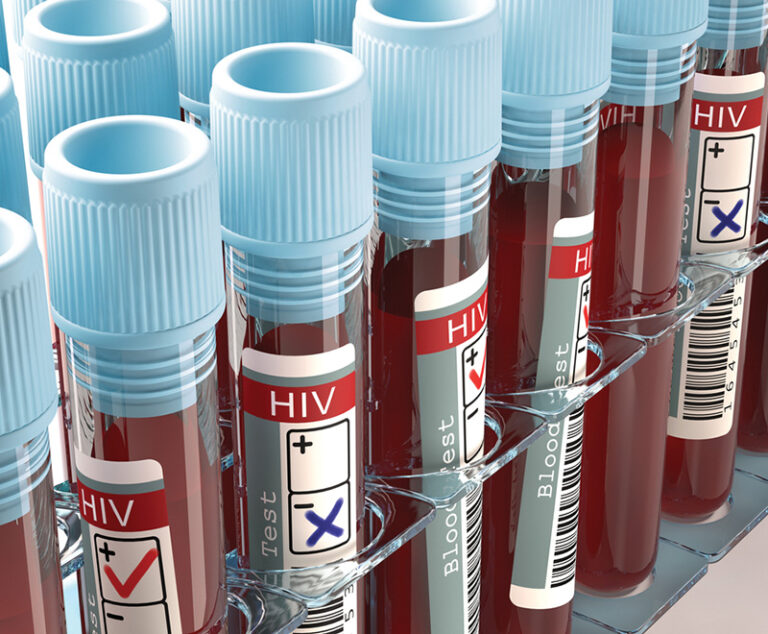
An Emory University-led research collaboration has been awarded a five-year $23.8 million grant from the National Institutes of Health (NIH) to fast-track research to cure HIV infection or put it in permanent remission.
A report from the U.S. Department of Health and Human Services (HHS) has found that considerable increases in the use of telehealth helped maintain some healthcare access during the COVID- 19 pandemic,
Novartis has received U.S. Food and Drug Administration (FDA) approval for its Cosentyx (secukinumab) to treat children and adolescents with enthesitis-related arthritis and psoriatic arthritis.