Industry News
Research, Science & Manufacturer Updates
University of Arizona Health Sciences researchers have received a $13.1 million grant from the National Institute on Aging to continue studies aimed at rejuvenating the immune system of older people to improve health throughout the lifespan.
The U.S. Department of Health and Human Services and the General Services Administration have announced new guidance recommending that all federal facilities across the nation include overdose reversal medications in their safety stations on site.
The U.S. Food and Drug Administration has authorized the use of Eli Lilly & Co.’s Zepbound for adults with obesity and moderate to severe obstructive sleep apnea, a common condition where a person struggles to breathe properly during sleep.
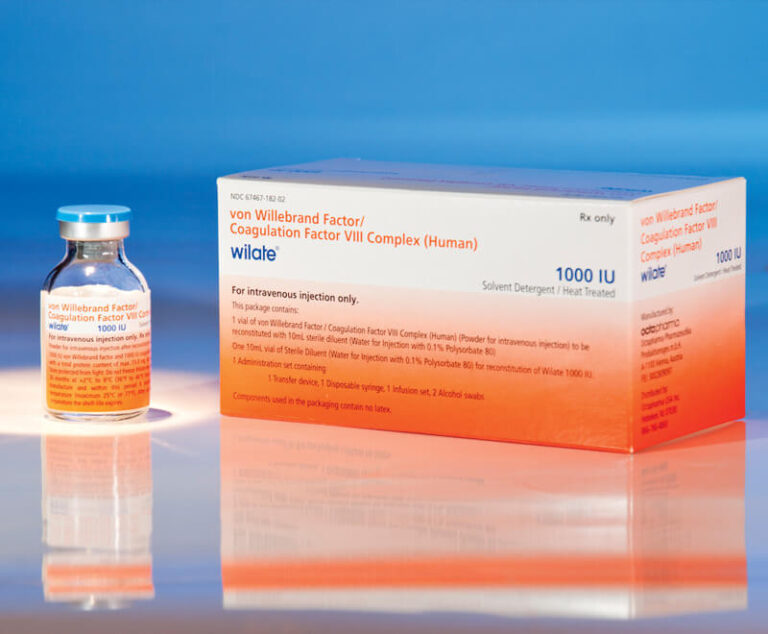
Octapharma USA's wilate, von Willebrand factor (VWF)/coagulation factor VIII complex (human) lyophilized power for solution for intravenous injection, has been given expanded approval by the U.S. Food and Drug Administration (FDA) for routine prophylaxis to reduce the frequency of bleeding episodes in adults and children aged 6 and older with any type of von Willebrand disease (VWD).
In a study, researchers found that fSCIG 10% was more effective in preventing CIDP relapse than placebo, supporting its potential use as maintenance CIDP treatment.
A team of Australian investigators conducted a retrospective cohort study to determine whether there is a significant benefit in more severely affected cases regularly infused with albumin.
An international early-phase clinical trial has found a "two-for-one" cancer immunotherapy, tebotelimab, is potentially more effective and at least as safe as standard immunotherapies.
A Phase I/II study evaluating the safety, tolerability and immunogenicity of Pfizer and BioNTech's mRNA-based combination vaccine candidates for influenza and COVID-19 among healthy adults 18 to 64 years of age show positive topline results.
Pfizer's PENBRAYA, a vaccine for meningococcal groups A, B, C, W and Y, has been approved by the U.S. Food and Drug Administration (FDA).
The U.S. Food and Drug Administration (FDA) has approved Celltrion's Zymfentra, a subcutaneous injection formulation of its infliximab Remsina, for maintenance therapy in adults with moderately to severely active ulcerative colitis and Crohn's disease following treatment with an infliximab administered intravenously.
The U.S. Food and Drug Administration (FDA) has approved Wezlana (ustekinumab-auub) as a biosimilar to and interchangeable with Stelara for adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy; active psoriatic arthritis; moderately to severely active Crohn's disease; and moderately to severely active ulcerative colitis.
The U.S. Department of Health and Human Services announced nine grant awards $1 million each for up to five years to support existing multidisciplinary Long COVID clinics across the country.