Industry News
Research, Science & Manufacturer Updates
The World Health Organization has published its first guideline for Ebola virus disease therapeutics, with new strong recommendations for the use of two monoclonal antibodies.
The U.S. Department of Health and Human Services will provide approximately $11 million to support the first U.S.-based fill and finish manufacturing of JYNNEOS, a vaccine approved to prevent smallpox and monkeypox.
In a Phase II trial of participants with hepatic disease associated with congenital alpha1-antitrypsin deficiency, subcutaneous administration of fazirsitran, an investigational RNA interference therapeutic, reduced both serum and hepatic levels of the mutant hepatotoxic AAT protein Z-AAT, with concurrent improvements in enzymatic and histological markers of liver function.
Subcutaneous administration of marstacimab, an investigational human monoclonal antibody targeting tissue factor pathway inhibitor, resulted in a roughly 10-fold lower annualized bleeding rate in 26 hemophilia patients than comparable control individuals in previous clinical trials who were treated on-demand with recombinant factor replacement therapy.
Dutch investigators conducted a systematic review and meta-analysis of studies on the effectiveness of IVIG treatment of this specific population.
Sanofi's fitusiran significantly reduced bleeding in a Phase III multinational study in adults and adolescents with hemophilia A or B, with or without inhibitors.
NIAID has launched an early-stage clinical trial to evaluate an investigational preventive vaccine for Epstein-Barr virus (EBV).
A new study shows treatment with IVIG shows promise for children and young adults with Down syndrome regression disorder (DSRD).
Despite technical challenges, some companies are working on making a combination COVID and flu vaccine.
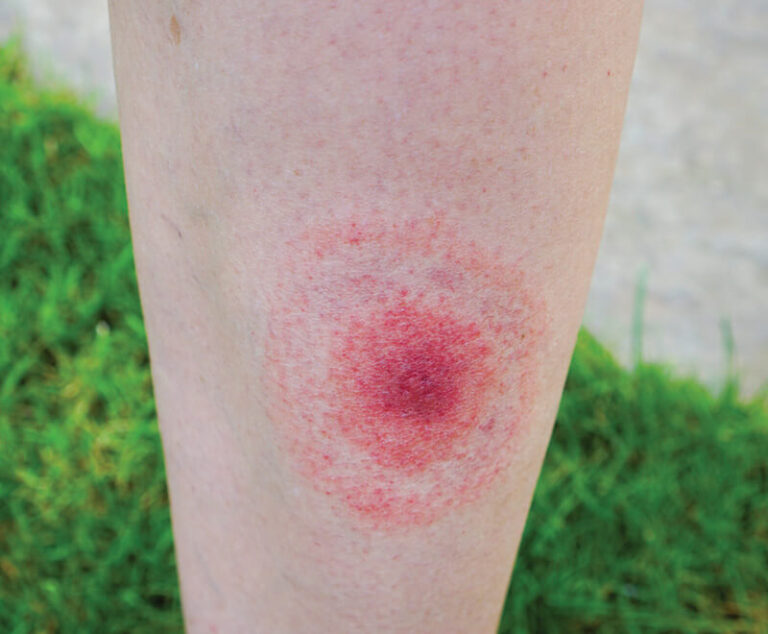
New data from a Phase II clinical trial of Valneva SE and Pfizer Inc.’s Lyme disease vaccine candidate, VLA15, shows it produced strong immune responses, prompting preparations for a Phase III study in the third quarter of 2022.
NIAID has launched a Phase I clinical trial evaluating three experimental HIV vaccines based on a mRNA platform — a technology used in several approved COVID-19 vaccines.
A clinical trial of the Vaxina (the CF33-hNIS virus) designed to kill cancer cells has begun.