Industry News
Research, Science & Manufacturer Updates
The U.S. Food and Drug Administration (FDA) has authorized the first over-the-counter combination COVID-19 and influenza test, the Healgen Rapid Check COVID-19/Flu A&B Antigen Test, outside of emergency use.
FDA has approved and granted emergency use authorization for updated mRNA COVID-19 vaccines (2024-2025 formula) to include a monovalent (single) component that corresponds to the Omicron variant KP.2 strain of SARSCoV-2.
The U.S. Department of Health and Human Services (HHS) has negotiated lower prices for 10 of the most expensive and widely used drugs covered under Medicare, which is expected to save the Medicare program $6 billion in the first year and reduce out-of-pocket costs for beneficiaries by $1.5 billion.
An investigational, individualized neoantigen therapy, with personalized encoded mRNA, has demonstrated potential to enable patients’ immune systems to target cells that cause cancer.
The U.S. Food and Drug Administration (FDA) has approved the first needle-free alternative to the EpiPen.
FDA has approved Octapharma USA's Fibryga (fibrinogen [human] lyophilized powder for reconstitution) for fibrinogen replacement in bleeding patients with acquired fibrinogen deficiency.
CMS issued a proposed rule that announces and solicits public comments on proposed policy changes for Medicare payments on or after Jan. 1, 2025.
FDA has published new draft guidance on informed consent that lines up with revisions to the Common Rule made in 2017, offering up-to-date recommendations on starting the process with the sharing of essential clinical trial information in ways that patients can understand.
SAMHSA released the results of the 2023 National Survey on Drug Use and Health (NSDUH), which shows how people living in the United States reported their experience with mental health conditions, substance use and pursuit of treatment.
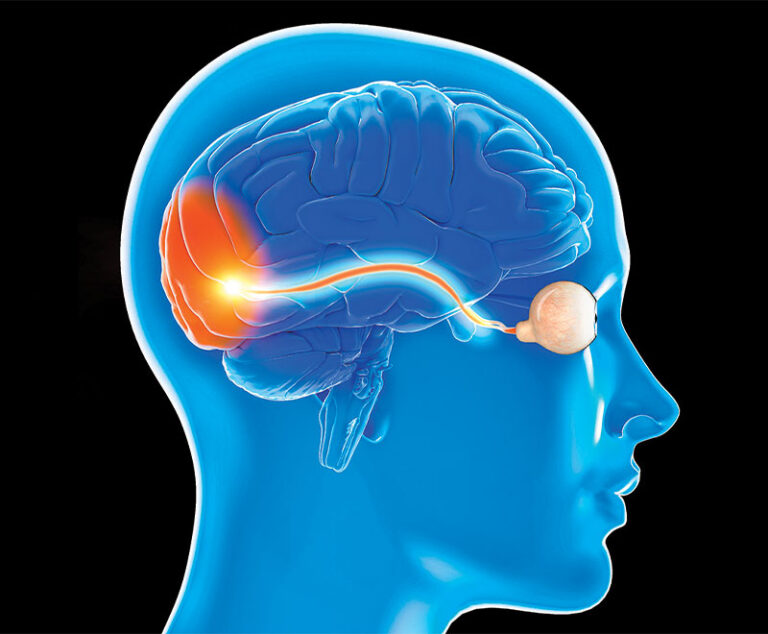
A meta-analysis and systematic review compared the efficacy of corticosteroids (CS) alone against CS combined with intravenous immune globulin (IVIG) or CS combined with plasmapheresis (PP) in patients with steroid-resistant optic neuritis.
In a Phase III, double-blind, three-way crossover trial, sebetralstat given on-demand for treatment of hereditary angioedema (HAE) attacks was shown to result in faster times to the start of symptom relief, reduction in attack severity and complete attack resolution than placebo treatment.
Scientists at Harvard Medical School have developed a simple nasal spray, made of harmless ingredients, that can protect people against flu, colds and COVID-19 with near-100 percent success, and it costs just $25.