Next-Generation Primary Care: How “Going to the Doctor” Is Changing

The future of healthcare is being driven by digital transformations and emerging technology that provide preventive, personalized and predictive medicine.
Scientists Develop $25 Nasal Spray That Is 99% Effective Against Colds, Flu and COVID-19

Scientists at Harvard Medical School have developed a simple nasal spray, made of harmless ingredients, that can protect people against flu, colds and COVID-19 with near-100 percent success, and it costs just $25.
Immune Globulin and Prophylactic Antibiotics Provide Similar Efficacy in Treating Hypogammaglobulinemia Secondary to Hematological Malignancy
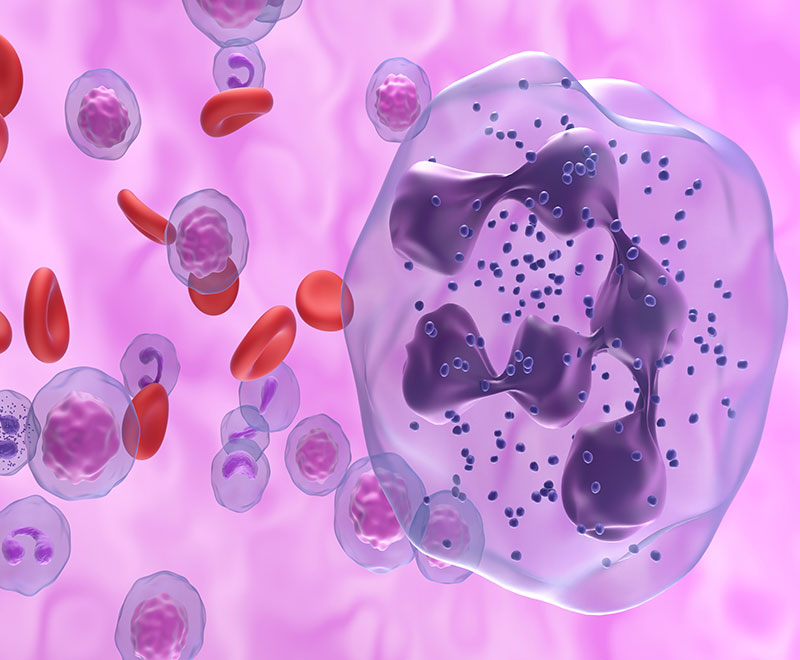
Immune globulin replacement and prophylactic antibiotics are commonly used to prevent infections in patients with secondary hypogammaglobulinemia due to hematological malignancies but have never been directly compared.
Update on Treating Neutropenia
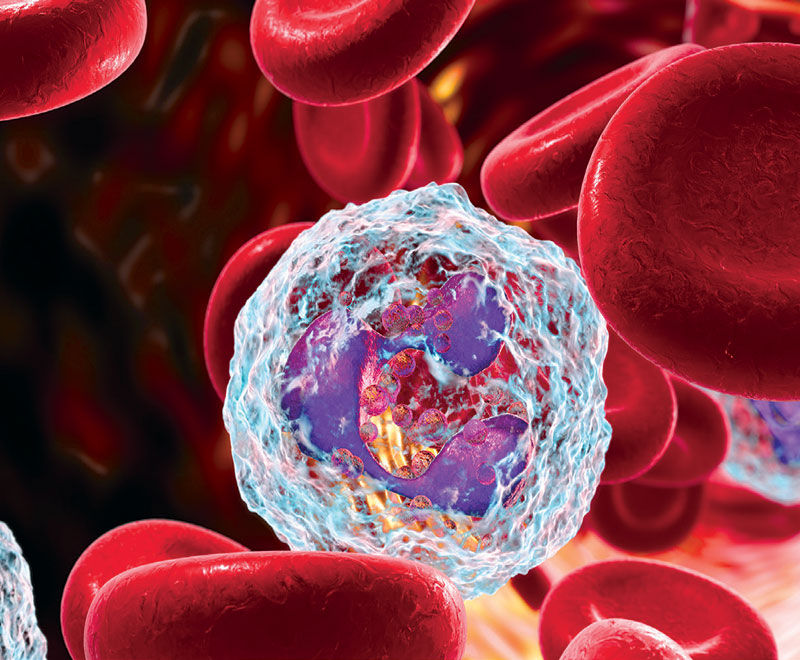
While neutropenia can be a life-threatening condition, physicians have many tools to treat it.
New Vaccine Could Lower ‘Bad’ Cholesterol by as Much as 30 Percent
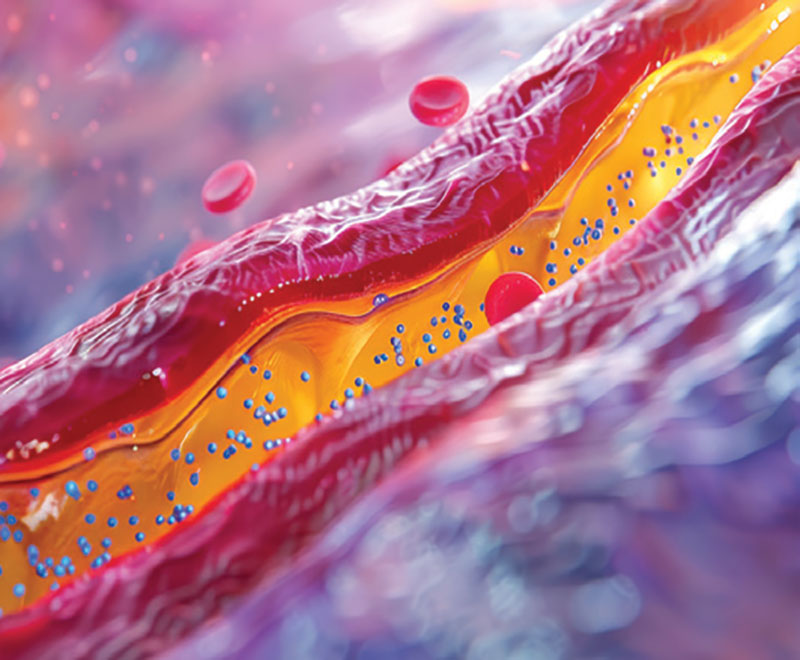
A new vaccine currently in development can effectively and affordably lower levels of “bad’ cholesterol in the body, a health problem that affects almost two in five adults in the U.S.
World’s First Gene Therapies Approved to Treat Sickle Cell Disease
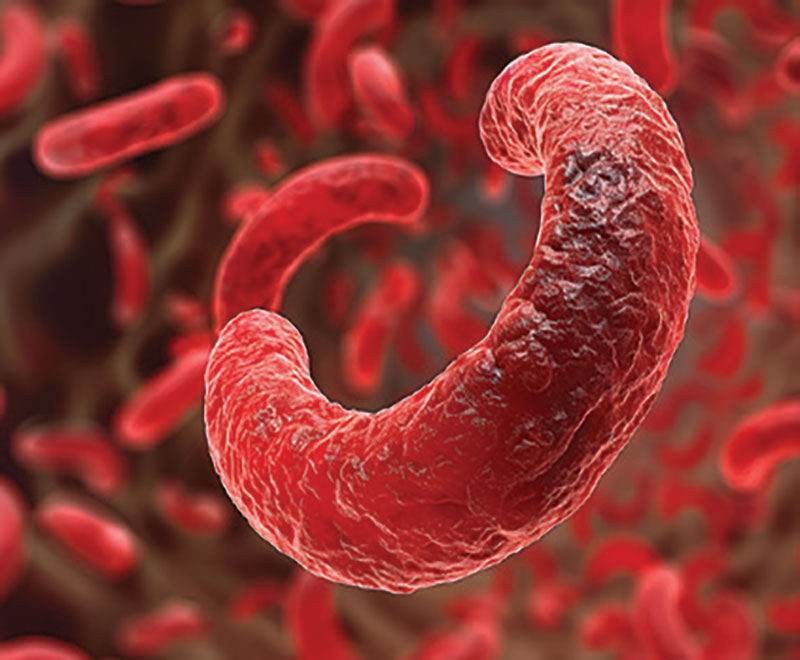
The U.S. Food and Drug Administration has approved two treatments, Casgevy and Lyfgenia, representing the first cell-based gene therapies for the treatment of sickle cell disease in patients 12 years and older.
Hizentra Vials to Be Discontinued and Replaced with Prefilled Syringes

CSL Behring is discontinuing all sizes of Hizentra vials in the U.S. by the end of September 2024.
Discovery Could Predict Immunotherapy Response in Melanoma

Researchers have discovered a rare type of immune cell that may predict how likely some patients with skin cancer will respond to immunotherapy treatment.
First Cancer Patients Receive mRNA Therapy in Clinical Trial
Imperial College Healthcare NHS Trust has enrolled the first United Kingdom (UK) patients who have received an experimental messenger RNA (mRNA) therapy — a type of immunotherapy treatment called mRNA-4359 — in its Phase I/II clinical trial.
FDA Expands Approval to wilate for Prophylaxis in All Types of VWDr
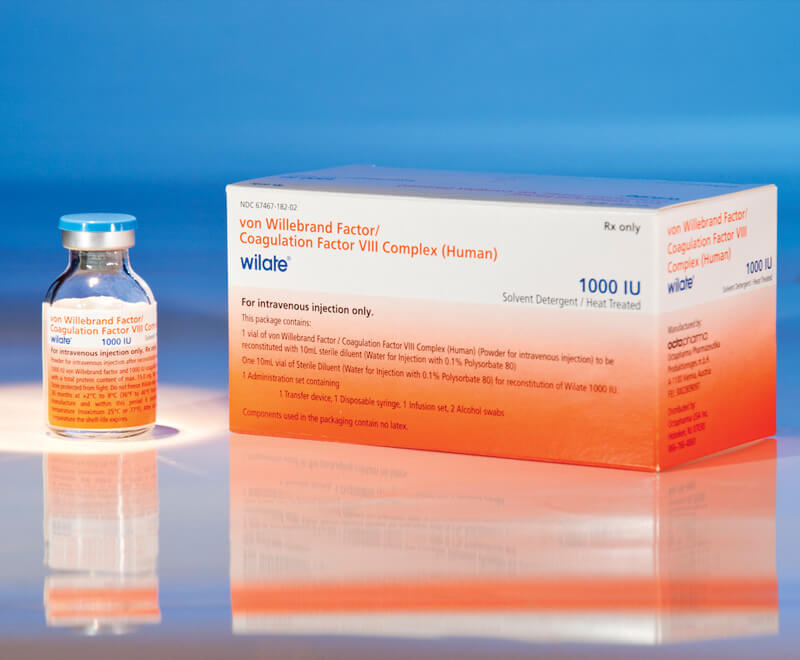
Octapharma USA’s wilate, von Willebrand factor (VWF)/coagulation factor VIII complex (human) lyophilized power for solution for intravenous injection, has been given expanded approval by the U.S. Food and Drug Administration (FDA) for routine prophylaxis to reduce the frequency of bleeding episodes in adults and children aged 6 and older with any type of von Willebrand disease (VWD).