University of Toledo Receives NIH Funds for Fungal Infection Research
A University of Toledo immunologist has received a federal grant to study how a type of cell unexpectedly discovered in the oral cavity helps the body fight off fungal infections.
Study Finds No Connection Between Antibiotic Use and Autoimmune Disease in Children
A groundbreaking retrospective cohort analysis of more than four million children conducted at Sungkyunkwan University in South Korea offers compelling evidence that there is no significant association between early antibiotic exposure and heightened risk of autoimmune diseases in children.
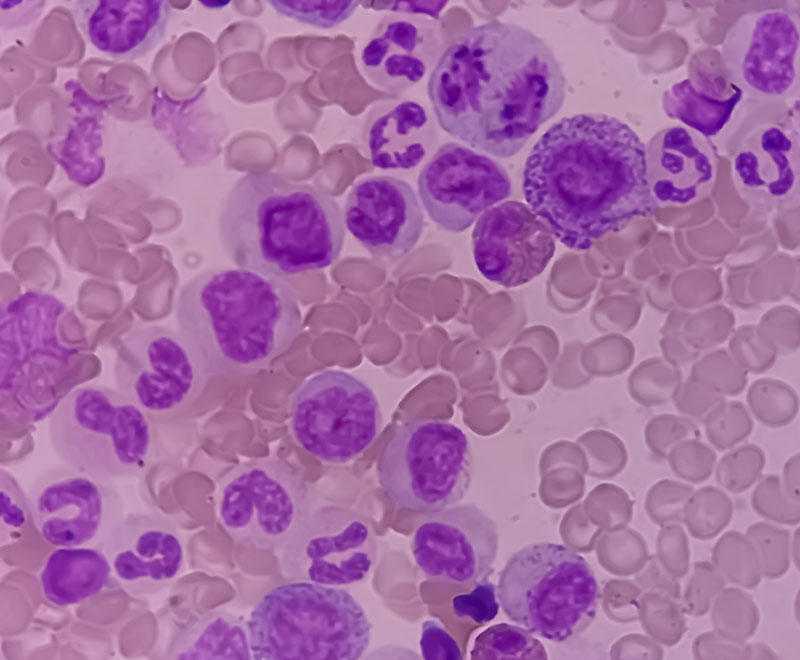
Study Finds Lymphoma Accelerates Aging of Immune Cells and Tissues
A new study reveals lymphoma can accelerate the biological aging of the immune system and other tissues.
NIH Changes Terms and Conditions Governing Federal Grants

The National Institutes of Health announced a significant change to the terms and conditions governing federal funding applicable to all NIH grants, cooperative agreements and other awards, including future awards.
New Vaccine Shows Promise Against Pancreatic Cancer
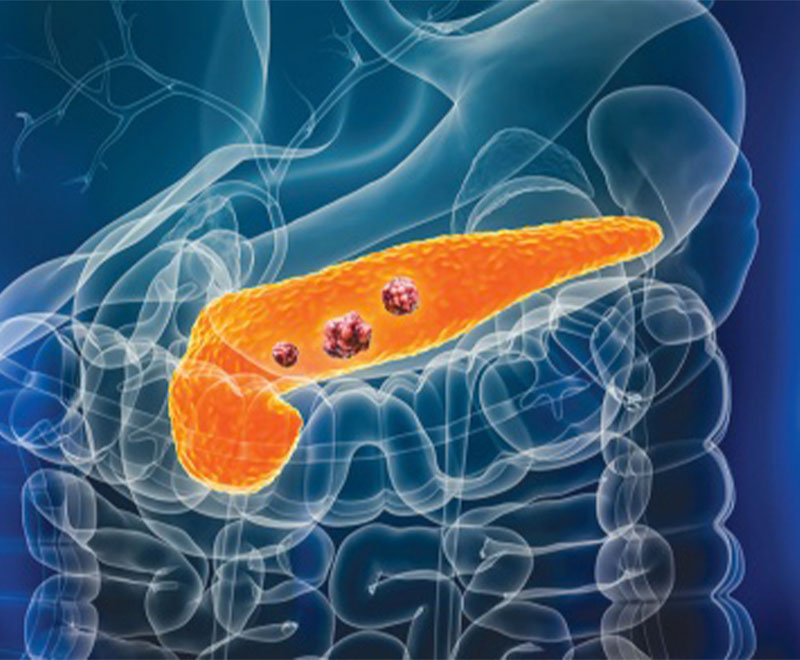
Early clinical and preclinical results are showing that an experimental mRNA and nanoparticle vaccine produced measurable immune responses against pancreatic cancer, and that in small patient groups, those immune responses correlated with delayed recurrence or prolonged survival.
HHS Reinstates Childhood Vaccine Safety Task Force

The U.S. Department of Health and Human Services has reinstated the Task Force on Safer Childhood Vaccines, a long-dormant federal panel created by Congress to oversee the safety and quality of children’s vaccines.
FDA Requires Major Changes to Opioid Pain Medication Labeling
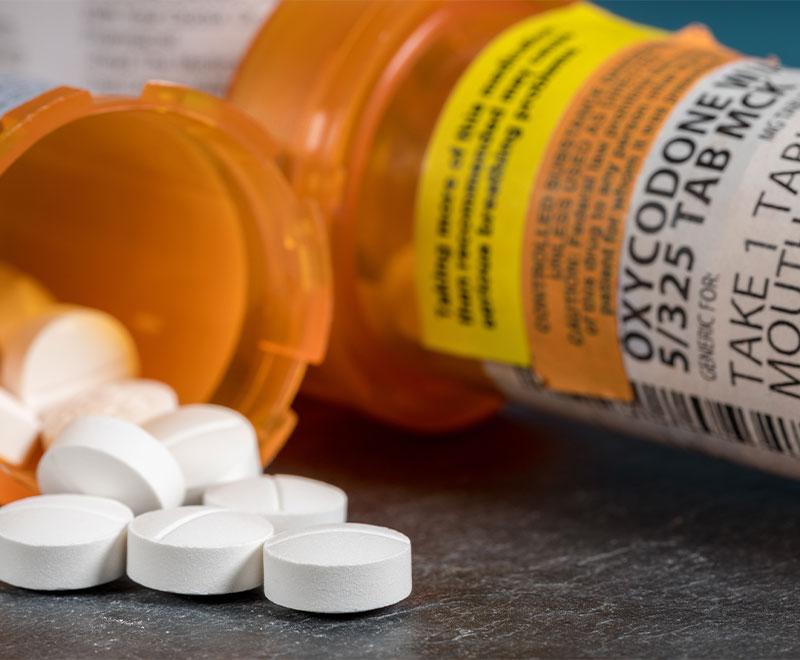
The U.S. Food and Drug Administration is requiring safety labeling changes to all opioid pain medications to better emphasize and explain the risks associated with their long-term use.
Argenx Is Conducting a Phase IV Study to Assess Efgartigimod Ph20 SC to Treat Adult CIDP Patients

Argenx is conducting a Phase IV clinical study to evaluate how adults with chronic inflammatory demyelinating polyneuropathy transition from intravenous immune globulin treatment to efgartigimod PH20 SC.
HHS Cuts Funding for mRNA Vaccine Research

The U.S. Department of Health and Human Services (HHS) has begun a coordinated wind-down of its mRNA vaccine development activities under the Biomedical Advanced Research and Development Authority (BARDA).
FDA Fast Tracks Immunotherapy for HER2-Positive Breast Cancer Recurrence

The U.S. Food and Drug Administration has granted fast track designation for Greenwich LifeSciences’ lead immunotherapy candidate GLSI-100 in HLA-A*02–positive, HER2-positive breast cancer patients who have completed standard HER2-targeted therapy.