FDA Approves Argenx’s Vyvgart to Treat MG
The U.S. Food and Drug Administration (FDA) has approved Vyvgart (efgartigi-mod) to treat generalized myasthenia gravis (gMG) in adults who test positive for the anti-acetylcholine receptor (AChR) antibody.
FDA Approves Avacopan to Treat Rare Autoimmune Disease

The U.S. Food and Drug Administration (FDA) has approved Chemo- Centryx Inc.’s Avacopan, sold under the brand name Tavneos, to treat antineutrophil cytoplasmic antibody-associated vasculitides.
FDA Implements New Warnings for Cigarette Packages and Advertisements

The U.S. Food and Drug Administration (FDA) issued a final rule to require new health warnings on cigarette packages and in cigarette advertisements, which feature textual statements with photo-realistic color images depicting some of the lesser-known, but serious health risks of cigarette smoking, including impact to fetal growth, cardiac disease, diabetes and more.
FDA Approves Uplizna to Treat Rare Autoimmune Disease of the CNS

The U.S. Food and Drug Administration (FDA) has approved inebilizumab-cdon (Uplizna) to treat neuromyelitis optica spectrum disorder (NMOSD) in adult patients with the anti-AQP4 antibody.
FDA Approves Biosimilar to Rheumatoid Arthritis Drug
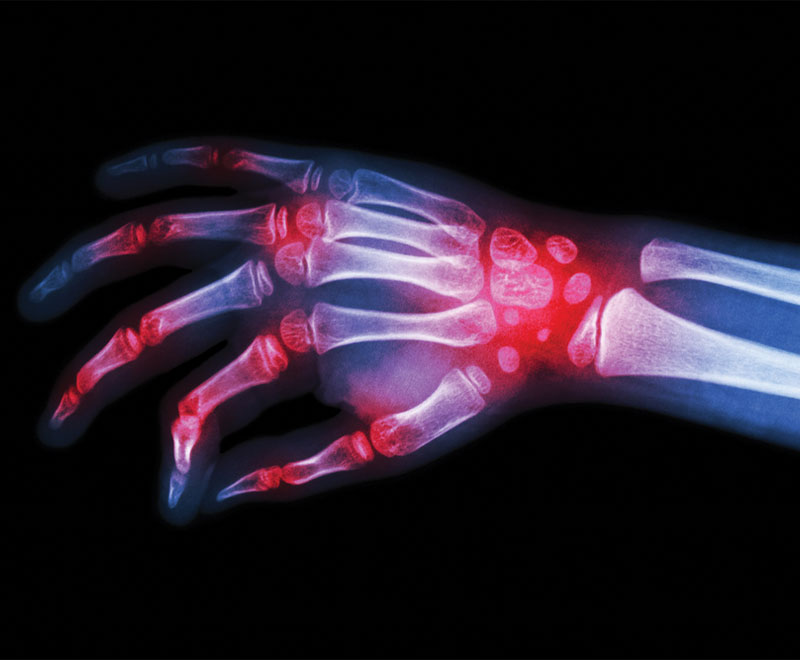
Amgen’s biosimilar to Johnson & Johnson’s rheumatoid arthritis drug, Remicade, has been approved by the U.S. Food and Drug Administration.
FDA Campaign Designed to Help Consumers Use New Food Label
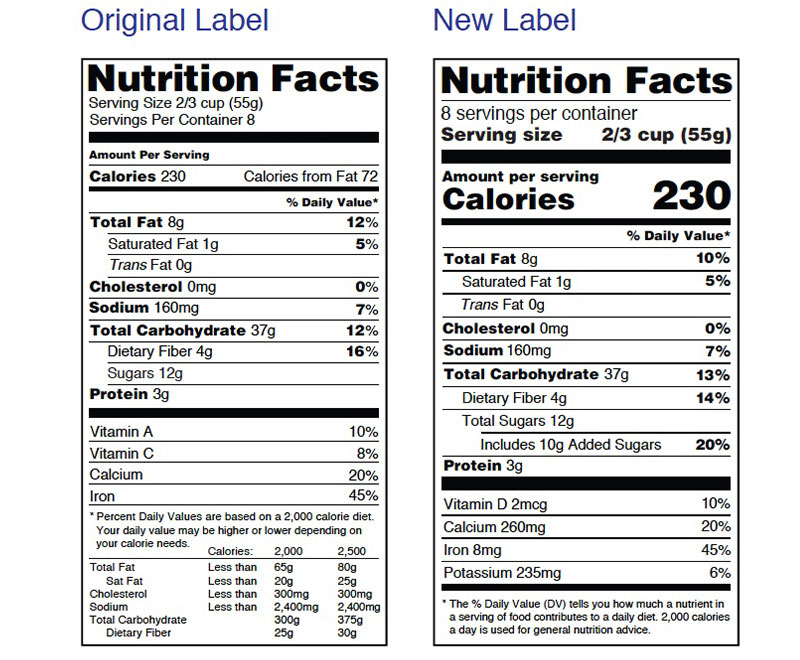
The U.S. Food and Drug Administration (FDA) has launched a campaign to help consumers use the new Nutrition Facts label that appears on packaged foods to maintain healthy dietary practices.
FDA Expands Indication for Octapharma’s WILATE to Hemophilia A

The U.S. Food and Drug Administration (FDA) has approved WILATE for treatment of adults and adolescents with hemophilia A for routine prophylaxis.
FDA Approves Sandoz’s Ziextenzo as 24th Biosimilar

The U.S. Food and Drug Administration (FDA) has approved Ziextenzo (pegfilgrastim-bmez), the 24th biosimilar approval in the U.S.
FDA Approves Octaplas to Treat Pediatric Patients Who Require Multiple Coagulation Factor Replacement
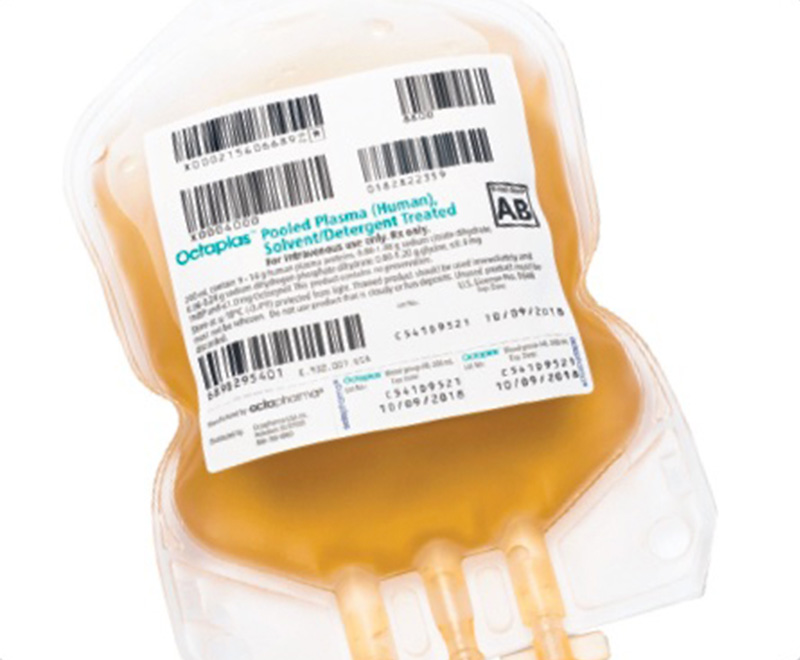
A revised product label for Octapharma USA’s Octaplas (pooled plasma [human] solvent/detergent treated solution for intravenous infusion) to treat critically ill pediatric patients who require replacement of multiple coagulation factors has been approved by the U.S. Food and Drug Administration (FDA).
FDA Issues Final Guidance on Biosimilar Interchangeability

The U.S. Food and Drug Administration (FDA) released guidelines on the studies companies need to conduct to show their biosimilar is interchangeable with a biologic.
