The Future of Cord Blood
Once thought to be a useless byproduct, umbilical cord blood is being used to treat more than 80 diseases today, and research indicates, in the future, it may be used to treat conditions far beyond just those that affect the blood and immune systems.
- By Ronale Tucker Rhodes, MS
A ONCE-DISCARDED birth byproduct, umbilical cord blood (UCB) has the potential to save thousands of lives each year in the U.S. For people born with life-threatening blood and immune system diseases, the best treatment is a bone marrow or blood cell transplantation from a related or unrelated donor or cord blood unit.1 Until recently, bone marrow transplantation has been the standard, as not much thought was given to UCB.
Beginning in the 1980s, doctors proposed it might be useful for treating some diseases. It was then that scientists realized UCB contained blood (haematopoietic) stem cells (HSCs), which can produce all other cells found in blood, including red blood cells, white blood cells and platelets. It is now known HSCs are responsible for maintaining blood production throughout life.2,3
Today, more than 20 years after the first successful UCB stem cell transplant, cord blood is changing lives with 13 percent of transplant patients receiving UCB donated to public cord blood banks.3,4 But, uses of cord blood are still in the beginning stages, and researchers believe this substance could radically transform medical strategies over the next few decades.
Harvesting Cord Blood
The closed technique is the most common method of harvesting cord blood stem cells, and it poses no risk to the mother or baby. This method is similar to drawing blood from a person. After a baby is born, cord blood is left in the umbilical cord and placenta, and then extracted by inserting a needle into the umbilical vein on the part of the cord that is still connected to the placenta. The typical amount removed is one to five ounces, and it takes less than 10 minutes to perform.2,5
Historically in Western countries, the umbilical cord was clamped and cut between 10 seconds and 15 seconds after birth. Now, more health organizations are recommending waiting for a period of time to clamp and cut a newborn’s umbilical cord after birth,6 thus decreasing the amount of cord blood that can be collected. These recommendations correlate with the World Health Organization’s (WHO) recommendation in 2012 that the umbilical cord should not be clamped earlier than necessary. Specifically, WHO recommends late cord clamping (performed approximately one to three minutes after birth) for all births.7
Recent research shows delayed cord clamping can benefit the newborn by allowing more blood to move from the placenta into the newborn, thereby increasing the child’s iron and hemoglobin levels and reducing the risk of iron deficiency during infancy, without increased risk to the mother. But, that means less blood is left in the umbilical cord, raising the question of whether there is enough cord blood to harvest. Fortunately, only approximately 50 mL of blood is necessary for cord blood storage, which is just a portion of the approximately 200 mL of blood contained in the placenta and umbilical cord. So, if cord clamping is delayed by one minute, about 80 mL of this blood is transferred into the infant, leaving more than enough to be stored in a cord blood bank. And, even if clamping is delayed by three minutes, only approximately 100 mL will have gone into the baby.8 Thus, such delayed clamping and cord blood collection are compatible.
Current Uses of Cord Blood
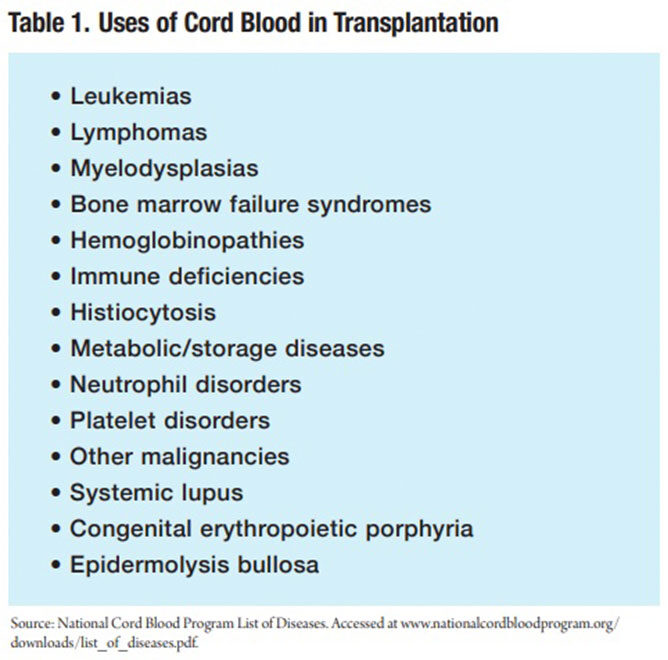
Cord blood is approved by the U.S. Food and Drug Administration (FDA) only for use in hematopoietic stem cell transplantation (HSCT) procedures, which are performed in patients with disorders affecting the hematopoietic (blood forming) system.9 To date, HSCT using cord blood has treated more than 80 different diseases. The most commonly treated disease category is leukemia, followed by inherited diseases of red blood cells, the immune system, metabolic abnormalities and others (Table 1).10 “Cord blood is useful because it is a source of stem cells that form into blood cells,” explains Keith Wonnacott, PhD, chief of the cellular therapies branch in FDA’s Office of Cellular, Tissue and Gene Therapies. “Cord blood can be used for transplantation in people who need regeneration, that is, ‘regrowth,’ of these blood-forming cells.”9
Cord blood can be used to treat the child from whom it was harvested, or to treat that child’s first- or second-degree relatives when it is stored in private cord banks. And, it can be used to treat individuals unrelated to the child after it has been donated to a public cord blood bank.9
Advantages and Limitations
There are a number of advantages to cord blood transplantation over bone marrow transplantation. For one, it is easy to collect. And, since it is donated in advance, routine testing is complete, and if a match is found, it can be reserved immediately with confirmatory human leukocyte antigen (HLA) typing and any special testing usually completed in five days. Therefore, unlike bone marrow, there is no need to take time to locate a possible volunteer to see if he or she is willing to donate.11
A significant advance is that studies have shown cord blood transplants can be performed even when the donor and recipient are partially matched, whereas bone marrow transplants require a perfect match in most cases. As such, a relatively small cord blood donor pool can support most patients’ needs. For example, the New York Blood Center’s National Cord Blood Program (NCBP) estimates a national inventory of 150,000 cord blood units would provide acceptable matches for at least 80 percent to 90 percent of U.S. patients.11
What’s more, the immune cells in cord blood are less likely than those in bone marrow from unrelated donors to cause graft versus host disease, which occurs when the transplanted cells attack the patient’s own tissues. In addition, cord blood is less likely to transmit infectious diseases such as Epstein-Barr virus and cytomegalovirus (CMV) that can be potentially lethal for transplant recipients. In fact, CMV is carried as a latent virus by approximately half of the population, whereas less than 1 percent of infants are born with CMV.11
For ethnic minorities, cord blood is particularly important. Because there are differences in the frequency of HLA types among ethnic groups, patients have a much more difficult time finding an unrelated bone marrow donor. The problem is simply numerical since minority groups have smaller numbers from which to draw potential donors. This is particularly true of African-Americans who make up only 12 percent of the U.S. population. In fact, epidemiological estimates indicate at least three times as many African-American volunteer bone marrow donors than Caucasian donors would be needed for AfricanAmerican patients to have a chance that equals that of Caucasian patients to find a match in the same bone marrow donor registries. The same problem is true for many Hispanic and Asian patients, who tend to have ancestors from more than one ethnic group. At the NCBP, 54 percent of U.S. patients transplanted have been non-Caucasian, 16 percent of whom have been African-Americans.12 And, at the Be The Match Registry, a listing of potential marrow donors and donated cord blood units, 28 percent of cord blood transplants in 2017 were for patients of color.4
Worth noting, a disadvantage of cord blood is that while it is a rich source of HSCs, the volume collected in one cord blood unit is fixed and relatively small, which means it contains fewer HSCs than a customizable bone marrow donation. For instance, the average total nucleated cell dose (number of nucleated cells per kilogram of a patient’s weight) in a cord blood graft is less than about one-tenth that of the average bone marrow graft.13 Because adults are larger and need more HSCs than children, a transplant containing too few HSCs may fail or could lead to slow formation of new blood in the body in the early days after transplantation. Thankfully, clinical trial results have shown double cord blood transplants (from two different donors) to be very successful.
Additionally, researchers have tried to increase the total number of HSCs obtained from each umbilical cord by collecting additional blood from the placenta. They are also studying ways to expand the number of HSCs from cord blood in labs so a single cord blood donation could supply enough cells for one or more HSC transplants. Known as ex-vivo expansion, this process has shown mixed results in many preliminary clinical trials. Some suggest ex-vivo expansion reduces the length of time for new blood cells to appear in the body after transplantation, but adult patients still need blood from two umbilical cords.14
Further limitations of cord blood must be considered, including that not all information about diseases carried in the infant’s blood is available as some genetic diseases may not be apparent in the child for months or years, and will not be found or even suspected by current screening methods. Also, cord blood from a newborn infant will not be available for an additional donation of cells, whereas with bone marrow transplants, the donor can be asked to make an additional donation.13
Banking on Cord Blood
While cord blood banks are essential to increasing patient access to transplant, the method in which cord blood is stored can be controversial. As mentioned previously, there are two main methods of storage — public and private — as well as a third known as hybrid. Public cord blood banks store cord blood donations for public use free of charge. Private cord blood banks store cord blood units for private use by individual families for a fee. And, hybrid cord blood banks offer both public and private cord blood banking services. According to BioInformant, a stem cell market research firm, as of May 2018, there are 53 cord blood banks in the U.S.: 27 private, 23 public and three hybrid.15
Public cord banks make donations available to anyone who needs them. In addition, the banks may use the donated cord blood for research. Patients who wish to donate cord blood to a public bank must talk with their doctor and then make arrangements with a cord blood bank. Because public banks pay for everything, including collecting, testing and storing UCB, cord blood donation is not possible in every hospital. In participating hospitals, the blood left in the umbilical cord and placenta is collected and tested. Cord blood that meets standards for transplant is stored at the public bank until needed by a patient. (It is not saved for the family making the donation.) After the cord blood unit arrives at the public bank, it is checked to ensure it has enough blood-forming cells for a transplant. If there are too few cells, it may be used for research to improve the transplant process for future patients, to investigate new therapies using cord blood or discarded. It is also checked to ensure it is free from contamination. Then, the tissue is typed and listed on the registry of the C.W. Bill Young Cell Transplantation Program, also called the Be The Match Registry, which is searchable to find a matching marrow donor or cord blood unit for a transplant patient. Cord blood donations are kept frozen in a liquid nitrogen freezer to be available if the unit is selected as a match for a patient needing a transplant.16
The U.S. Congress mandates all patients in need of a transplant have access to bone marrow, blood cell and UCB transplants. The C.W. Bill Young Cell Transplantation Program was authorized in December 2005 after enactment of the Stem Cell Therapeutic and Research Act of 2005. That act was then amended by the Stem Cell Therapeutic and Research Reauthorization Act of 2010 and 2015. In fall, 2006 and 2012, Be The Match was awarded key contracts to carry out the work mandated in the C.W. Bill Young Cell Transplantation Program, including the contract to serve as the nation’s Cord Blood Coordinating Center. The stem cell act of 2015 helped patients by creating the National Cord Blood Inventory, whose goal is to collect and store an additional 150,000 cord blood units for patients in need of transplant and for research to continue improving the success of transplants. The act also allows for funding for bone marrow and UCB transplantation and research through Be The Match.17
At private banks, cord blood cells remain the property of the donor in case the child or a relative is faced with a serious health issue in the future. Collection and processing are the same as at a public bank. Private banks provide the service for a fee that covers the cost of collection and processing, as well as annual storage. There are two forms of payment that affect the cost: The first, determined by the bank, is an initial payment of approximately $1,500 or more, and it covers only the first year. After that, the donor is required to pay approximately $100 or more annually for storage. The second is cheaper, but the donor pays upfront for storage for 20 years.18
According to Save the Cord Foundation, hybrid banks are private banks that also operate a public donation program. Hybrid banks help make donations possible regardless of location since many hospitals do not provide access for donations to public banks. Costs for these programs are often covered in part by the private side of the business. Due to the costs involved in running a public donation program, though, many hybrid banks limit the number of donations they can accept each year.19
A Controversial Cure?
While there is little doubt public storage of cord blood is essential to providing patients access to transplants, many medical professionals and researchers question the usefulness of private cord blood storage.20 The American Academy of Pediatrics (AAP) encourages parents to keep their child’s cord blood if a family member has already been diagnosed with a stem-cell-treatable disease, but the chances of a child actually needing his or her stored cord blood stem cells is anywhere between one in 1,000 and one in 200,000, according to studies cited by the American College of Obstetricians and Gynecologists and AAP.21 Another study at the University of California, San Francisco, found there is a 0.04 percent chance of a baby requiring a transplant of his or her own stored stem cells to treat a disease and a 0.07 percent chance the baby’s sibling will require a stem cell transplant from the baby’s stored cord blood.20
Private banks’ marketing materials, on the other hand, often place the odds at one in 2,700. “Researchers are constantly discovering new treatments using stem cells,” says Gerald Maass, executive vice president of corporate development for Cryo-Cell, a private bank in Clearwater, Fla. And, another major bank’s website claims: “Should cord blood prove successful in treating heart disease, the lifetime probability of being diagnosed with a disease treatable by cord blood will increase from one in 100 to one in two.”21
But, the opposing argument that questions cord blood stem cells’ usefulness for the donor has merits. According to a 2011 study published in the Journal of Assisted Reproductive Medicine, “If cord blood from an infant donor has an inherited hematologic, immunologic or genetic disorder, then cord blood may not be used to expect a cure for the same disease in the same recipient. Therefore, families with recognized genetic diseases should be made aware of these issues.” This means if a child has leukemia or if he or she is diagnosed with a genetic illness such as an immune deficiency, the genetic mutations that led to that child’s disease is in the DNA of his or her cord blood and is, therefore, unusable.20
Also, a controversial issue surrounding private banks remains. As mentioned previously, few cord blood transplants are given to adults because most units don’t contain enough stem cells to treat anyone weighing more than 90 pounds, according to Joanne Kurtzberg, MD, program director of the division of pediatric blood and marrow transplantation at Duke University Medical Center. And, says Mary Halet, manager of cord blood operations for the Center for Cord Blood at the National Marrow Donor Program, approximately 75 percent of the units donated to public banks are discarded or used in research because they don’t contain enough stem cells for transplants.21
Lastly, since the procedure is relatively new, no one knows how many years cord blood can be stored in liquid nitrogen for the cells to remain viable. However, according to NCBP, its earliest units were stored in 1993, and after checking the viability of cells in units that will not be used for transplantation, it has not detected any deterioration in the quality of the cells in those stored for up to 16 years. And, units stored for up to 13 years have been used in transplants, and the outcomes have been similar to those of newly collected units.22
Unlocking Cord Blood’s Potential
Looking to the future, the use of cord blood stem cells may extend beyond regeneration of healthy blood and immune systems. Several reports suggest cord blood may contain other types of stem cells that can produce specialized cells that do not belong to the blood. To name a few, studies continue on cord blood transplants to treat spinal cord injury, heart attacks, stroke, Alzheimer’s, Parkinson’s disease, type 1 diabetes, cerebral palsy, traumatic brain injury and acquired hearing loss, among others. Following are highlights from some of these studies:
• In a study published in June 2010, newborn cord blood stem cells improved the neurologic function of rats after an acute spinal cord injury. The rats experienced a significantly improved recovery of locomotor function over a six-week period compared to untreated rats. And, six weeks after treatment, the injured area was noticeably smaller in the treated animals.23
• The American Heart Association recently published results of a study that intravenously infused umbilical cord stem cells in 30 patients aged 18 years to 75 years who had stable heart failure. Participants were given the stem cell therapy or a placebo. When analyzing the results, researchers compared the two and noted those who received the stem cell infusion showed sustained and significant fourfold improvement in the hearts’ ability to pump blood, reported a greater quality of life and suffered no adverse effects as a result of the therapy.24
• In a study assessing the safety and feasibility of a single intravenous infusion of non-HLA-matched, ABO-matched, unrelated allogeneic UCB in adult stroke patients, 10 participants with acute middle cerebral artery ischemic stroke were enrolled. UCB units were matched for blood group antigens and race but not HLA, and infused three to nine days poststroke. The adverse event (AE) profile over a 12-month postinfusion period indicated the treatment was well tolerated with no serious AEs directly related to the study product. Study participants were also assessed using neurological and functional evaluations, including the modified Rankin Score (mRS) and National Institutes of Health Stroke Scale (NIHSS). At three months posttreatment, all participants had improved by at least one grade in mRS and by at least four points in NIHSS, relative to baseline. Together, these data suggest a single intravenous dose of allogeneic non‐HLA-matched human UCB cells is safe in adults with ischemic stroke, and support the conduct of a randomized, placebo‐controlled Phase 2 study.25
Just recently, scientists at Duke University in North Carolina began investigating the effect of cord blood on sufferers of adult ischemic stroke. In the study, 100 patients between 18 years and 90 years of age will undergo UCB transfusions between three and 10 days after their stroke. They will then be monitored for results.26
• In a study conducted at Stanford University School of Medicine, researchers injected into mice either cord blood plasma or blood from people aged between 19 years and 24 years or 61 years and 82 years. When the older mice received human UCB plasma every fourth day for two weeks, their memory, learning and hippocampal function improved notably, as well as their ability to navigate through a complex maze. Plasma from older people, on the other hand, was no help at all, while young adult plasma only induced an intermediate effect. After realizing something in the UCB was making the old brains act younger, the scientists discovered a protein called TIMP2, an important protein that vanishes as humans get older. Injecting TIMP2 by itself into elderly mice largely duplicated the beneficial effects of UCB. The Stanford team had already proved that young blood can reverse some of the signs of aging in mice but have never shown it could restore learning and memory.27
• A study conducted in 2015 examined whether transplanting UCB stem cells had positive, therapeutic effects on rat models with Parkinson’s disease. They found the cord blood stem cells “significantly improve the motor deficits of the Parkinson’s disease rats.” According to the researchers, results suggest using cord blood stem cells “would have a significant impact on future strategies for Parkinson’s diseases treatment.”28
• A study conducted at the University of Illinois, Chicago, found stem cells from cord blood “re-educated” the immune system T cells of people with type 1 diabetes so their pancreas started producing insulin again, thereby reducing the amount of insulin they needed to inject. The small, open-label, Phase 1/Phase 2 study recruited 15 patients with type 1 diabetes aged 15 years to 41 years with a diabetic history ranging from one to 21 years. All but three of the patients (controls) underwent stem cell educator therapy once. Controls underwent a sham treatment in which they received no educated cells. The researchers checked the patients’ progress at four, 12, 24 and 40 weeks after therapy. Six of the patients who had the therapy had some residual beta cell function (moderate type 1 diabetes) and the other six had no residual beta cell function (severe type 1 diabetes). Results showed the median daily dose of required insulin was down by 38 percent at week 12 for the six patients with moderate diabetes and by 25 percent for the patients with severe diabetes. There was no change in required insulin dose for the controls. Levels of C-peptide continued to improve at 24 weeks and were maintained to the end of the study at 40 weeks.29
• An infusion of cells from a child’s own UCB appears to improve brain connectivity and motor function in children with spastic cerebral palsy, according to a randomized clinical trial. The placebo-controlled, Phase 2 trial included 63 children with varied types and severities of spastic cerebral palsy, a condition usually caused by brain damage before or at birth. Children who received one intravenous dose of at least 25 million stem cells per kilogram of their body weight saw improvements in motor function a year later. The improvements were greater than those typically observed for children of similar age and condition, and exceeded the gains made by children who received a lower dose of cells or a placebo.30
• In a study of the safety and efficacy of a novel therapeutic trial with UCB and concomitant recombinant human erythropoietin conducted in three cases of severe traumatic brain injury in rehabilitation, researchers found participants showed improvements during follow-up periods in various aspects. Patient 1 demonstrated improvements in motor and cognitive function, and diffusion tensor images showed increased nerve fibers. Patient 2 displayed improvements in activities of daily living. And in Patient 3, neurogenic fever vanished and brain PET revealed increased glucose metabolism at basal ganglia, thalami and cerebellum. There were no serious adverse events in any of the patients.31
• A Phase 1 clinical trial is seeking to enroll 10 children between 6 weeks to 6 years of age with less than 18 months of hearing loss with spoken language the intended end point. The trial, inspired by a Duke University study of 30 patients suffering from acquired hearing loss, shows cord blood transplants can be a treatment for sensorineural hearing loss due to mucopolysaccharidosis. The findings demonstrate hearing loss can be easily measured with a unit called ABR that is a count of functioning hair cells. If the transplant shows an improvement in ABR, it suggests the functioning hair cells are growing in number. In a previous trial, human UCB stem cells were used to treat hearing loss in a mice model, which resulted in replacement of hair cells. According to James Baumgartner, MD, a pediatric neurosurgeon at Florida Children’s Hospital, “There was a pretty significant improvement, in particular if the stem cell transplant was done before 25 months of age.”32
Expanding Uses for Diseases
It’s been only approximately three decades since UCB was found to have lifesaving properties that can treat blood and immune system disorders. Since then, cord blood transplants have successfully treated thousands of patients afflicted with more than 80 diseases each year. Although cord blood transplants have many advantages over bone marrow transplants, including easy and painless collection, immediate use, partial match between donors and patients, lower rejection and disease transmission rates, and a larger pool of donors for ethnic minorities, they also have limitations that must be considered, including low volumes of HSCs, current inability to detect genetic diseases in the UCB and the unavailability of a second donation. Nevertheless, while cord blood banks and donations continue to expand, they will surely be needed since research continues to show promise for the use of cord blood stem cells to treat an ever-growing list of diseases.
References
- Health Resources and Services Administration. Blood Cell General FAQ. Accessed at bloodcell.transplant.hrsa.gov/about/general_faqs/index.html.
- Umbilical Cord Blood Is Saving Lives. Health Blog, Nov. 2, 2012. Accessed at healthyone.org/umbilical-cordblood-is-saving-lives.
- Cord Blood Stem Cells: Current Uses and Future Challenges. Avensblog,Dec. 8, 2015. Accessed atwww.avens online.org/blog/cord-blood-derived-natural-killer-cells-in-multiple-myeloma-patients.html.
- Be The Match. Cord Blood Is Changing Lives. Accessed at bethematch.org/support-the-cause/donate-cordblood/cord-blood-is-changing-lives.
- How Is Cord Blood Collected? CorCell, Dec. 29, 2014. Accessed at www.corcell.com/blog/how-is-cordblood-collected.
- Wait to Cut Umbilical Cord, Experts Urge. CNN Wire, March 3, 2017. Accessed at fox40.com/ 2017/03/03/wait-to-cut-umbilical-cord-experts-urge.
- World Health Organization. Optimal Timing of Cord Clamping for the Prevention of Iron Deficiency Anaemia in Infants. Accessed at www.who.int/elena/titles/full_recommendations/cord_clamping/en.
- Cord Blood Banking vs. Delayed Cord Clamping: Can I Do Both? CorCell, Dec. 11, 2013. Accessed at www.corcell.com/blog/cord-blood-banking-vs-delayed-cord-clamping.
- U.S. Food and Drug Administration. Cord Blood: What You Need to Know. Accessed at www.fda.gov/ForConsumers/ConsumerUpdates/ucm405558.htm.
- National Cord Blood Program. What Is Cord Blood Used For? Accessed at www.nationalcordbloodprogram.org/qa/what_is_treated.html.
- National Cord Blood Program. What Are the Advantages of Cord Blood? Accessed at www.nationalcordbloodprogram.org/qa/what_are_advantages.html.
- National Cord Blood Program. Why Is Cord Blood Important for Ethnic Minorities? Accessed at www.nationalcordbloodprogram.org/qa/what_is_significance.html.
- NationalCord Blood Program. Are There AnyUnfavorable Aspects ofCord Blood? Accessed at www.nationalcordbloodprogram.org/qa/how_is_it_collected.html.
- EuroStemCell. Cord Blood Stem Cells: Current Uses and Future Challenges — Current Research: Blood Diseases. Accessed at www.eurostemcell.org/cord-blood-stem-cells-current-uses-and-future-challenges.
- Hildreth C. List of U.S. Cord Blood Banks — Public, Private and Hybrid. BioInformant, May 24, 2018. Accessed at bioinformant.com/us-cord-blood-banks.
- Health Resources and Services Administration. Donating Umbilical Cord Blood to a Public Bank. Accessed at bloodcell.transplant.hrsa.gov/cord/options/donating/index.html.
- Be The Match. Federal Cord Blood Legislation. Accessed at bethematch.org/support-the-cause/donatecord-blood/cord-blood-is-changing-lives/federal-cord-blood-legislation.
- Cord Blood Bank. Accessed at www.cordbankingblood.com.
- Save the Cord Foundation. What Is a Hybrid Bank? Accessed at www.savethecordfoundation.org/why-choose-a-hybrid-bank.html.
- Is Cord Blood Banking Worth It? The Great Debate. Yahoo Parenting, May 16, 2015. Accessed at www.yahoo.com/news/is-cord-blood-banking-worth-it-the-great-debate-119041849782.html.
- Teng, J. The Cord Blood Controversy. Accessed at www.selfgrowth.com/articles/the-cord-blood-controversy.
- National Cord Blood Program. How Long Does Cord Blood Remain Viable? Accessed at www.nationalcordbloodprogram.org/qa/how_long.html.
- Potential Stem Cell Therapies. Stemell, Oct. 2, 2017. Accessed at stemell.com/2017/10/02/potential-stemcell-therapies.
- Umbilical Cord Stem Cells Can Repair Heart Disease. Cells4Life, Sept. 29, 2017. Accessed at cells4life.com/2017/09/umbilical-cord-stem-cells-repair-heart.
- Laskowitz, DT, Bennett, ER, Durham, RJ, et al. Allogeneic Umbilical Cord Blood Infusion for Adults with Ischemic Stroke: Clinical Outcomes from a Phase I Safety Study. Stem Cells Translational Medicine, May 12, 2018. Accessed at stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.18-0008.
- Umbilical Cord Blood Used to Treat Stroke in Adults. Cells4Life, July 4, 2018. Accessed at cells4life.com/ 2018/07/umbilical-cord-blood-used-treat-stroke-adults.
- Knapton, S. Umbilical Cord Blood from Babies Could Help Bring Back Memory for Dementia Patients. The Telegraph, April 19, 2017. Accessed at www.telegraph.co.uk/science/2017/04/19/umbilical-cord-bloodbabies-could-help-bring-back-memory-dementia.
- Chen, C, Duan, J, Shen, A, et al. Transplantation of Human Umbilical Cord Blood-Derived Mononuclear Cells Induces Recovery of Motor Dysfunction in a Rat Model of Parkinson’s Disease. Journal of Neurorestoratology, 4 April 2016, Volume 2016:4 Pages 23-33. Accessed at www.dovepress.com/transplantation-ofhuman-umbilical-cord-blood-derived-mononuclear-cell-peer-reviewed-article-JN.
- Paddock, C. Type 1 Diabetes Reversed with Stem Cells from Cord Blood. Medical News Today, Jan. 11, 2012. Accessed at www.medicalnewstoday.com/articles/240160.php.
- Khanna, S. Umbilical Cord Blood Improves Motor Skills in Some Children with Cerebral Palsy. Duke Department of Pediatrics, Oct. 30, 2017. Accessed at pediatrics.duke.edu/news/umbilical-cord-bloodimproves-motor-skills-some-children-cerebral-palsy.
- Min, K1, Song, J, Lee, JH, et al. Allogenic Umbilical Cord Blood Therapy Combined with Erythropoietin for Patients with Severe Traumatic Brain Injury: Three Case Reports. Restorative Neurology and Neuroscience, 2013;31(4):397-410. Accessed at www.ncbi.nlm.nih.gov/pubmed/23515110.
- India, C. Umbilical Cord Blood Stem Cells to Treat Acquired Hearing Loss in Kids. CordLife, June 4, 2017. Accessed at www.cordlifeindia.com/blog/umbilical-cord-blood-stem-cells-treat-acquired-hearing-loss-kids.
