Subcutaneous Immune Globulin Maintenance Therapy for CIDP: An Idea Whose Time Has Come
- By Keith Berman, MPH, MBA
MOST OFTEN DIAGNOSED in people between 40 years and 60 years of age, chronic inflammatory demyelinating polyneuropathy (CIDP) is a relatively rare immune-mediated peripheral nervous system disorder that results in variable loss of grip strength and upper and lower limb weakness. Patients may find themselves unable to get up from a sitting position, maintain balance or handle small or delicate items. If left untreated, irreversible axonal damage can occur, with cumulative disability that eventually leads to wheelchair dependence in about one-third of patients.
While its exact mechanism of action remains unclear, intravenous immune globulin (IVIG) has consistently been shown in well-designed clinical trials to be effective in durably reducing disability in roughly one-half of affected patients.1,2 As maintenance therapy to prevent disease relapse, IVIG is preferred over corticosteroids, plasma exchange or immunosuppressive drug options.
But the benefits of long-term IVIG administration often come with significant downsides. Even after employing available strategies such as slowing the infusion rate or switching product brands, some patients suffer systemic reactions that can include headache, fatigue, fever, chills, hypotension, tachycardia, myalgia, lower-back pain, rash, flushing, nausea and vomiting. Particularly in patients with predisposing risk factors, IVIG administration has also been associated with serious systemic adverse events, including renal insufficiency and, in rare instances, thrombosis or anaphylactoid reactions. In the clinic or home setting, IVIG must be infused by a specially trained nurse, and the patient must adhere to a set scheduled infusion regimen.
As documented in several recent pivotal clinical trials, a potential solution for CIDP patients with these IVIG-related issues is the same one that works for many primary humoral immunodeficiency (PI) patients who require IgG replacement therapy: self-administered subcutaneous immune globulin (SCIG).
A recent investigation randomized 30 CIDP patient responders to IVIG for a switch to a corresponding total dose of SCIG administered thrice-weekly at home or to thrice-weekly subcutaneous saline. The SCIG group experienced a modest 5.5 percent mean improvement in isokinetic muscle strength, versus a 14.4 percent mean decline in the placebo group.3 More recently, a meta-analysis of eight studies comparing the efficacy and safety of IVIG and SCIG in patients with CIDP or multifocal motor neuropathy (MMN), another chronic inflammatory demyelinating neuropathy, found no significant differences in muscle strength outcome; SCIG therapy was associated with a significantly reduced risk of moderate and/or systemic side effects.4
In March 2018, based on results from the double-blind, placebo-controlled Phase 3 PATH trial,5 CSL Behring’s 20% SCIG product (Hizentra), approved in 2010 for the treatment of PI, became the first to secure an additional indication for the treatment of adults with CIDP as maintenance therapy to prevent relapse of neuromuscular disability and impairment. Another SCIG product already approved for PI, Shire’s HyQvia, is currently being investigated for use as CIDP maintenance therapy. By all accounts, SCIG is already gaining popularity among patients and physicians as the IgG maintenance treatment of choice.
“Maintenance SCIG therapy is a potential option for any CIDP patient who requires ongoing treatment, and is willing to learn how to self-administer the product at home,” said Leslie Vaughan, chief operations officer at NuFACTOR Specialty Pharmacy. But, she added, most patients who decide to switch to SCIG therapy from nurse-managed home or clinic-based IVIG infusions appear to be motivated by one or more of these reasons:
- Poor tolerance to systemic side effects during or shortly after IVIG infusion
- Poor venous access necessitating placement of a vascular access device
- A desire for more flexibility in scheduling infusions to minimize work/lifestyle conflicts
- A desire to be independent of the need for nurse-managed clinic or home infusion visits
Divided SCIG Doses Reduce Systemic Reactions
Following a typical 2 gram per kilogram (g/kg) induction dose of IVIG, most responders receive maintenance therapy infusions of greater than or equal to 1 g/kg of body weight every three to four weeks,* under the management of a nurse infusion specialist in the home or in the clinic setting. IVIG administration results in immediate (within six hours) or delayed systemic adverse reactions in roughly 5 percent to 15 percent of infusions, affecting as many as 20 percent to 40 percent of all patients.6 By contrast, across five case series evaluating SCIG in PI patients, the reported rates of systemic adverse reactions ranged between zero and less than 1 percent.7 The largest of these studies, monitoring 33,168 SCIG infusions in 158 patients, documented a systemic adverse reaction rate of just 0.3 percent: 100 mild and six moderate events in 28 patients with no severe or anaphylactoid reactions.8
“Patients on SCIG therapy experience far fewer systemic side effects such as headache, nausea, chills and fatigue because the IgG is administered subcutaneously in frequent, much smaller doses than IVIG,” explained Vaughan. While local swelling, itching, heat, pain and erythema reactions at the SCIG infusion site are common, they generally resolve within 12 hours to 24 hours without treatment and tend to diminish over time. These typically mild local reactions are rare with IVIG infusion.9
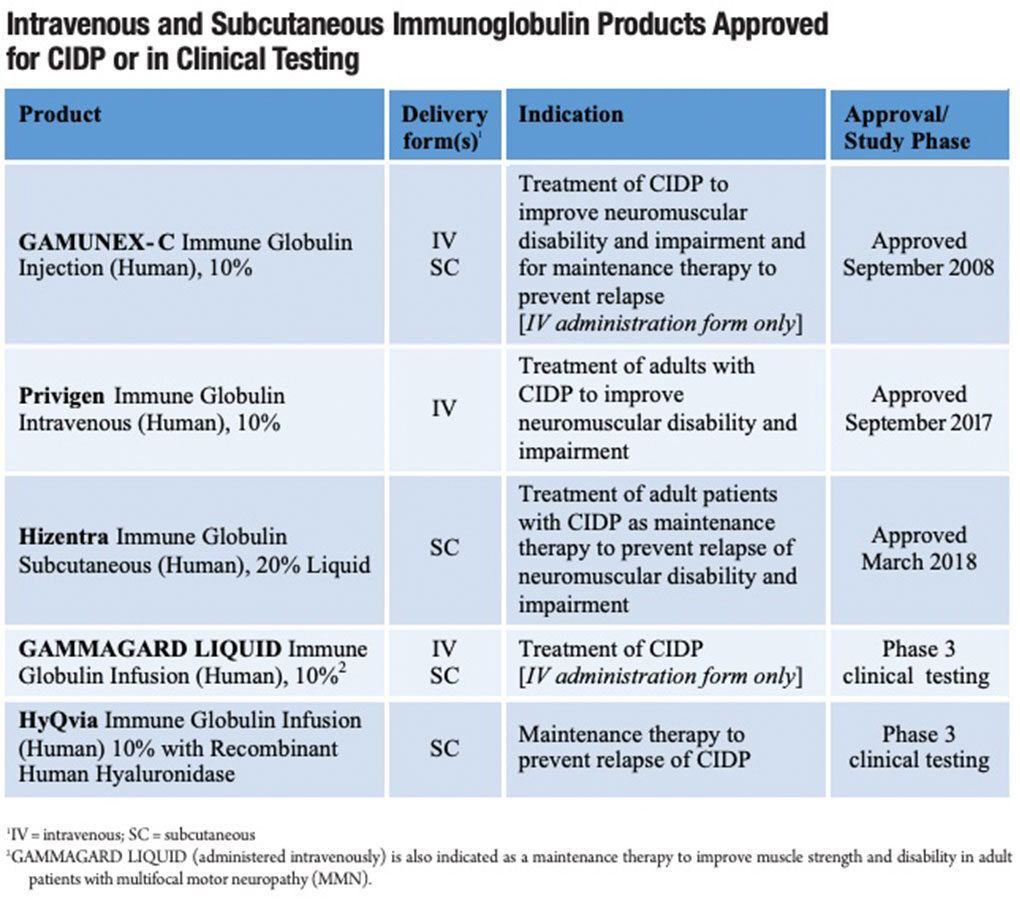
SCIG therapy delivers a similar quantity of IgG as IVIG over the same three or four-week period, but the peak serum IgG level is much reduced by dividing the IVIG dose into one or more doses a week; a common twice-weekly SCIG infusion schedule, for example, divides a monthly IVIG dose into eight much smaller doses. The serum IgG peak following each of these small subcutaneous infusions is additionally moderated by its relatively slow absorption into the bloodstream. Because the large IgG protein is unable to cross capillary endothelial walls to directly enter the circulation, it instead slowly transits through the lymphatic system.10 The serum IgG level peaks between 48 hours and 72 hours following an SCIG infusion.
A combination of small divided doses and slow absorption appears also to diminish the severity of the infrequent systemic events that occur with SCIG. Danish investigators recently examined two of the most common side effects of IVIG — headache and nausea — in 59 patients diagnosed with CIDP, MMN or postpolio syndrome treated with IVIG, and 27 CIDP and MMN patients treated with SCIG. Patients reported symptom severity on a visual analogue scale (VAS) from 0 mm to 100 mm. In the SCIG group, headache reached a median peak value of just 1 (range 0 to 13) mm at day six, versus a median peak value of 11 (range 0 to 96) in the IVIG group at day four. Nausea experience in the SCIG group had a stable median value of 0 (range 0 to 21) at all days, compared to a peak value of 3 (range 0 to 90) reached at day four in the IVIG group. For both headache and nausea, this reduced median severity favoring SCIG was highly significant (p <0.0001). Just as important, the peak severity experienced by any patient was also sharply lower in the SCIG group.11
A Better Alternative to Ports or Catheters
A small percentage of CIDP patients prescribed maintenance IVIG therapy either have pre-existing venous access problems or develop them with repeated peripheral intravenous access. Permanent indwelling venous catheters or infusion ports implanted under the skin were once a very popular means to resolve this venous access problem.12
Unfortunately, these venous access devices inherently present a significant risk of infection. Localized tissue reaction produced by these devices makes it easier for bacteria and other microorganisms to become established and develop into an active infection. Some types of infections, in particular colonization with Candida albicans, frequently require removal of the port or catheter. Skin bacteria can also gain entry to the port through the needle puncture site, then travel down the catheter lumen to the vein, potentially causing a systemic infection.
A second significant concern is the potential for ports or indwelling catheters to promote thrombus formation, amplifying the risk of a thromboembolic event rarely associated with administration of IVIG itself.13
Citing these known risks of infection and thrombosis, a recently published American Academy of Allergy, Asthma and Immunology practice policy statement recommended that “the placement of permanent central venous access [devices] solely for the purpose of IVIG administration should be discouraged,” particularly given the “growing availability of subcutaneous IgG infusion.”14
Reduced Wearing-Off Effect
Exogenous IG therapy is known to be effective only as long as the supplemental IgG serum level is maintained in the therapeutic range. Some patient responders on maintenance IVIG therapy experience a diminution in muscle strength over the days immediately preceding their upcoming scheduled IVIG infusion. This “wearing off” effect is attributable to a drop-off in serum IgG to below the therapeutic threshold level prior to the next scheduled IVIG infusion — a phenomenon that is averted by frequent IgG dosing used by patients on SCIG therapy.
In a recent crossover study, one-quarter of subjects who reported a preference for SCIG cited the advantage of less fluctuation in muscular strength than they experienced on IVIG therapy. This is unsurprising as small, frequent SCIG doses result in a more consistent serum IgG level, in particular a higher IgG trough level than the trough level shortly prior to the next IVIG infusion (Figure). While the problem of waning muscle strength in the days prior to the next IVIG infusion can also be addressed by increasing the IVIG dose or reducing the interval between IVIG infusions, both of these strategies have downsides that can be averted by switching to SCIG therapy.
Customizing the SCIG Infusion Regimen Is Key
Whether the product is IVIG or SCIG, CIDP patients on maintenance therapy are typically prescribed a total dose of at least 1 g/kg of IgG every three to four weeks. Thus, an 80 kilogram adult prescribed 1 g/kg of 20% SCIG product each four weeks must use an infusion pump to self-administer a total of 400 mL of fluid under the skin over that period. Prescribing instructions for Hizentra specify that, as tolerated, up to a maximum of 50 mL may be infused in each site (abdomen, thigh, upper arm or side of upper leg/hip). In a given session, patients can concurrently infuse their product through up to eight needles placed in different areas of the body. So, in theory, a patient able to tolerate 50 mL in a single infusion site could self-administer 100 mL in just one session each week using just two needles placed in two separate sites on the body. But for most CIDP patients, the maximum tolerated single-site infused volume is much lower than 50 mL.
To meet their prescribed weekly SCIG volume, patients face a choice: They can elect either to 1) increase the number of needles and sites they use in each infusion session, or 2) use fewer needles and increase the number of infusion sessions each week. While most patients settle on two to three infusions per week, “the balance between how many needles to use in a session versus how often to self-infuse is highly individual,” said Amy Ehlers, NuFACTOR Specialty Pharmacy’s director of pharmacy. “Patients need time to learn and become comfortable with the experience of self-administering SCIG before they decide what works best. If patients are pushed to try a lot of needles or volume in the early stages, some will rebel.”
Independence and Scheduling Flexibility
While relief from systemic side effects is an important reason patients cite for switching from IVIG to SCIG, for many patients, SCIG is also valued for the freedom it offers to self-treat on their own schedule, or for independence from reliance on nurses and other medical professionals. This has been documented in multiple PI patient studies, including a seminal 2006 investigation of the impact of SCIG on health-related quality of life (HRQoL) in 28 PI patients previously treated with IVIG in a clinic setting (group A) and 16 others previously on IVIG therapy at home (group B). 15 After switching to SCIG therapy, group A reported significantly less limitation in their work and daily activities, better general health and improved treatment satisfaction; more than 80 percent preferred the subcutaneous route, and 90 percent preferred the home treatment setting. Two-thirds of group B patients treated at home with IVIG followed by SCIG stated their preference for the subcutaneous route.
Results from a more recent IVIG-versusSCIG preference study in a CIDP patient cohort echo the PI study findings.3 Twenty of 29 CIDP patients who crossed over from effective IVIG therapy to SCIG indicated they preferred SCIG therapy. Sixteen of these 20 patients cited increased infusion scheduling flexibility as a reason. More stable strength, milder side effects and time savings were cited as reasons by five, three and two patients, respectively.
It is too early to speculate about what eventual proportion of CIDP patients who require chronic maintenance therapy will elect to switch to SCIG therapy in lieu of remaining on IVIG. Some CIDP patients are needle-phobic or are otherwise uncomfortable with the steps required to self-administer the drug. Others may have residual fine motor control deficits or other issues that preclude this option.
But the experience of the PI population might provide some insight about the prospects for SCIG as maintenance therapy for CIDP: Little more than a decade after the 2006 approval of the first SCIG treatment, SCIG is now the IgG replacement therapy of choice for more than one-half of PI patients in the United States.
*Some CIDP patients may require IVIG infusions as often as every two weeks or as infrequently as every eight weeks.
References
- Hughes RA, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol 2008 Feb;7(2):136-44.
- Léger JM, De Bleecker JL, Sommer C, et al. Efficacy and safety of Privigen in patients with chronic inflammatory demyelinating polyneuropathy: results of a prospective, single-arm, open-label Phase III study (the PRIMA study). J Peripher Nerv Syst 2013 Jun;18(2):130-40.
- Markvardsen LH, Debost JC, Harbo T, et al. Subcutaneous immunoglobulin in responders to intravenous therapy with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol 2013 May;20(5):836-42.
- Racosta JM, Sposato LA and Kimpinski K. Subcutaneous versus intravenous immunoglobulin for chronic autoimmune neuropathies: A meta-analysis. Muscle Nerve 2017 Jun;55(6):802-9.
- van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double blind, placebo-controlled, phase 3 trial. Lancet Neurol 2018 Jan;17 (1):35-46.
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev 2013 Jul;27(3):171-8.
- Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol 2004;112:1-7.
- Gardulf A, Andersen V, Bjorkander J, et al. Subcutaneous immunoglobulin replacement in patients with primary antibody deficiencies: safety and costs. Lancet 1995 Feb;345:365-9.
- Berger M. Subcutaneous IgG in neurologic diseases. Immunotherapy 2014;6(1):71-83.
- Supersaxo A, Hein WR and Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res 1990 Feb;7(2):167-9.
- Markvardsen LH, Christiansen I, Andersen H, et al. Headache and nausea after treatment with high-dose subcutaneous versus intravenous immunoglobulin. Basic Clin Pharmacol Toxicol 2015 Jun 12;117:409-12.
- Immune Deficiency Foundation. Are Infusion Ports Appropriate for Delivering Ig for Primary Immunodeficiency? Accessed 8/14/2018 at primaryimmune.org/treatment-information/immunoglobulintherapy/are-infusion-ports-appropriate-for-delivering-ig-forprimary-immunodeficiency.
- Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: A review of evidence. J Allergy Clin Immunol 2017;139:S1-46.
- Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol 2015;136(5):1185-1205.
- Nicolay U, Kiessling P, Berger M, et al. Health-related quality of life and treatment satisfaction in North American patients with primary immunedeficiency diseases receiving subcutaneous IgG self-infusions at home. J Clin Immunol 2006 Jan;26(1):65-72.