Myths & Facts: Kawasaki Disease
Educating physicians about this not-so-rare disease can curtail lifelong consequences that can occur if not diagnosed and treated early.
- By Ronale Tucker Rhodes, MS
NAMED AFTER Japanese pediatrician Tomisaku Kawasaki, Kawasaki disease (KD) is a rash/fever illness of early childhood in which coronary artery aneurysms (CAAs), sometimes fatal, may develop in up to 25 percent of untreated children. KD is a type of vasculitis, meaning it causes inflammation of the blood vessels. In 1967, Dr. Kawasaki first described 50 cases of KD in infants with persistent fever, accompanied by rash, lymphadenopathy, edema, conjunctival injection, redness and cracking of the lips, “strawberry tongue” and convalescent desquamation. However, during the 1960s, a significant controversy in Japan was whether the rash and fever sign/symptom complex was connected to subsequent cardiac complications in a number of cases. That connection was established in 1970 when the first Japanese nationwide survey of KD documented 10 autopsy cases of sudden cardiac death after KD. In 1974, the first English-language report of these KD patients was published by Dr. Kawasaki. The disease was independently recognized as a new and distinct condition in the early 1970s by pediatricians Marian Melish and Raquel Hicks at the University of Hawaii. Since that time, KD has become the leading cause of acquired heart disease among children in North America and Japan.1,2
The incidence of KD is highest in Japan with an annual rate of 130 to 140 per 100,000 children under 5 years of age. In comparison, the incidence of KD in the continental U.S. varies between nine and 20 per 100,000 children under 5 years of age, and for Japanese Americans living in Hawaii, the incidence is between 120 and 130 per 100,000 in children under 5 years of age.1
While an infectious agent is suspected, the actual cause of KD remains unknown. However, there has been significant progress toward understanding the natural history of the disease, and therapeutic interventions have been developed that halt the immune-mediated destruction of the arterial wall.1 Unfortunately, despite more than a half century of treating KD patients, a great deal of myths continue to circulate, resulting in delayed treatment that can prove fatal.
Separating Myth from Fact
Myth: Only Asian children are susceptible to KD.
Fact: While children of Asian or Pacific Islander descent have higher rates of KD, it can affect children of any race. In fact, KD has been diagnosed in more than 60 countries, including those in Asia, the Middle East, Latin America and Africa, as well as in North America and Europe.3
Myth: KD only occurs in children between the ages of 1 and 5 years.
Fact: KD more commonly affects children younger than 5 years old, and the majority of those are less than 2 years old. But, KD can be most severe in children under 1 year of age. In addition, KD can affect older children.4 In 2016, out of 5,440 KD-related hospitalizations in children under 18 years old, 3,935 involved children younger than 5 years old.1 KD also occurs more often among boys.4
Myth: KD is caused by a single biological factor that causes or contributes to the development of a disease or condition.
Fact: KD has been linked to a broad range of infectious agents, as well as environmental exposures, including carpet shampoo, genetic variants and meteorological patterns5 (it is more commonly seen in the winter and spring months4). However, the etiology of KD remains unknown, and it is believed that KD likely results from the interaction of one or more infectious agents and genetic factors. What is known is that KD is not contagious.
Because KD occurs when children are most at risk of infection (6 months to 5 years) and in winter months, when most childhood infections occur, epidemiological evidence strongly suggests an infectious trigger.5 Infections may cause the immune system to attack the blood vessel walls by mistake and cause inflammation. It is also suspected that KD might be the result of changes to certain genes or related to viral or bacterial infections.6
Myth: There is a link between KD and COVID-19.
Fact: No studies have found a link between KD and COVID-19. In fact, in Japan, a KD Surveillance Team reported that no association was observed between KD and COVID-19 during 2020.7 Rather, COVID-19 may cause a condition known as multi-system inflammatory syndrome (MIS-C)-19 with symptoms that resemble KD in some children.8
Myth: If a child presents with a viral infection, a diagnosis of KD is unlikely.
Fact: In a study published in the journal Pediatrics, a positive respiratory viral PCR, including for SARS-CoV-2, or presence of viral symptoms at the time of symptom presentation does not exclude a diagnosis of KD. In the study of 192 children with KD, 93 (41.9 percent) had a positive respiratory viral PCR, which did not correlate with gastrointestinal or respiratory symptoms. The researchers noted that a positive PCR for respiratory viruses, such as adenovirus, may represent asymptomatic shedding or latency in the upper airway.5
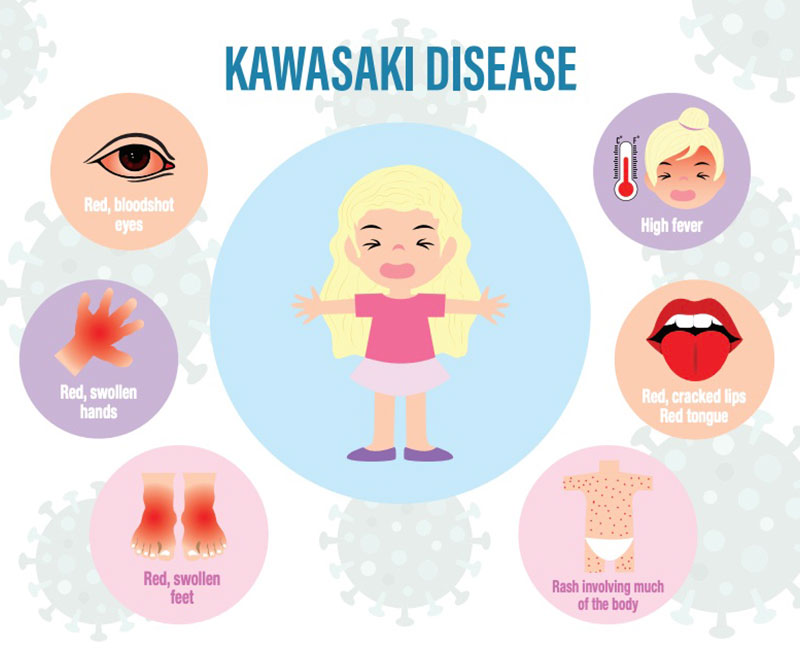
Myth: Children with KD always display all of the known symptoms.
Fact: Actually, children with KD do not all experience the same or all of the symptoms of KD. To diagnose KD, a child will present with a fever greater than 101 degrees Fahrenheit for five or more days, as well as with at least four of the following symptoms:
- A rash on the main part of the body or in the genital area
- An enlarged lymph node in the neck
- Very red eyes without a thick discharge
- Red, dry, cracked lips and a red, swollen tongue (strawberry tongue)
- Swollen, red skin on the palms of the hands and the soles of the feet
And, while KD occurs suddenly, its symptoms often occur in phases. The above are known as Phase 1 symptoms. Phase 2 symptoms include:
- Skin peeling on the tips of the fingers and toes
- Diarrhea
- Abdominal pain
- Joint pain
- Vomiting
During the third phase, the signs and symptoms will go away slowly, sometimes taking up to eight weeks. In addition, children may show symptoms of tiredness, irritability and low energy.5,9
Long-term complications include coronary artery lesions that can cause heart disease, meningitis and arthritis.
Some children may develop what is known as incomplete KD that happens if they experience a high fever for five or more days but have fewer than four of the symptoms needed for a KD diagnosis. However, it’s important to note that even children with incomplete KD are at risk of damage to their heart arteries.
There are no tests available to diagnose KD; it is diagnosed based on symptoms.9 However, doctors may order blood tests and a urine sample to rule out other diseases and common viruses that present with the same symptoms such as a recent strep or viral infection. Other diseases that should also be ruled out include scarlet fever, juvenile rheumatoid arthritis, Stevens-Johnson syndrome (a disorder of the mucous membranes), toxic shock syndrome, measles and some illnesses caused by ticks such as Rocky Mountain spotted fever.10
If a child meets the criteria for a KD diagnosis, a cardiology team will need to be consulted to perform a history, physical exam, an electrocardiogram to assess the electrical system of the heart and an echocardiogram (an ultrasound of the heart). Even if these studies are normal, the child will receive treatment based on clinical symptoms and lab work.4
Myth: The treatment window for intravenous immune globulin (IVIG), first-line treatment for KD, is 10 days.
Fact: Timely treatment of KD is imperative to reduce the risk of complications. The goal of treatment is to lower fever, reduce swelling and prevent heart damage. Treatment includes moderate to high-dose aspirin until fever subsides for up to eight weeks and IVIG, which contains antibodies to stop the immune system from attacking the blood vessels. IVIG is the most effective treatment for KD. Children should be treated with IVIG preferably within 10 days of the onset of symptoms, and the American Heart Association and American Academy of Pediatrics recommend starting IVIG ideally within seven days. However, even if the 10-day window has passed, children should still be treated if they have a fever or other signs of inflammation.11
For children who don’t respond to IVIG, corticosteroids may be an option.4 More recently, a study has shown that adding glucocorticoids to the initial highdose IVIG and aspirin regimen effectively reduced fever duration, length of hospital stay and incidence of coronary artery lesions (CALs) in children with KD who exhibited three or more risk factors for IVIG resistance. In that study, 236 IVIGsensitive cases and 38 IVIG-resistant cases were gathered, with 26 cases in the observation cohort. Following treatment, those resistant to IVIG indicated higher rates of CALs compared with the IVIG-sensitive group at all post-treatment intervals. Prior to treatment, the observation group had higher CAL incidence than the IVIG-sensitive group.
In addition, patients with three or more high-risk factors in the IVIGresistance group were deemed to be much higher than that of the IVIG-sensitive group. This was also the case in the observation group. In both the IVIG-sensitive and observational study groups, fever resolution times and duration of hospital stays were significantly reduced. According to the researchers, “this is especially notable given the more severe inflammatory reaction present in patients with KD who are IVIG-resistant than those who are IVIG-sensitive. Importantly, the treatment combination led to clinical improvement and CAL reduction in patients with KD without adverse effects related to glucocorticoids, a positive indication of safety.”12

Myth: All children should receive high-dose aspirin in addition to IVIG.
Fact: While aspirin has been used as a concomitant drug in the treatment of KD, in recent years, the role and optimal dose of aspirin remain controversial. In 2020, results were published of a study conducted to identify if the dose of aspirin in the acute phase of KD will facilitate development of a more appropriate treatment strategy in improving the outcome of KD. In the study, a total of 2,369 patients with KD were retrospectively analyzed and divided into three groups according to the aspirin dose: 510 in group 1 (20 to 29 mg/kg/ day), 1,487 in group 2 (30 to 39 mg/kg/ day) and 372 in group 3 (40 to 50 mg/kg/day). The differences in laboratory data, rate of IVIG resistance and coronary artery damage were compared among the groups.
Results showed there was no difference in the incidence of CAAs in group 1 compared with groups 2 and 3 (two weeks of illness: 2.94 percent vs. 1.90 percent vs. 3.36 percent; three to four weeks of illness: 1.94 percent vs. 2.32 percent vs. 2.65 percent). The risk for developing CAA was not reduced at two weeks of illness onset in groups 2 and 3 compared with group 1. Furthermore, the risk for developing CAA was not reduced at three to four weeks of illness onset in groups 2 and 3. There was no significant difference in the rate of IVIG resistance among the groups. Platelet levels after IVIG treatment in group 1 were significantly lower than those in groups 2 and 3. C reactive protein of the 30 to 40 mg/kg day group was slightly higher than the other two groups. The researchers concluded that aspirin at the lower dose of 20 to 29 mg/kg/day dose not increase the risk of coronary artery damage and IVIG resistance compared with the high dose of 30 to 50 mg/kg/day. In addition, the low dose may have a lower risk for a potential effect on liver function.13
Myth: Once KD is resolved with treatment, there is no longer a risk of cardiovascular disease.
Fact: While IVIG treatment can decrease the risk of cardiovascular disease, it does not eliminate the risk of developing CAAs. However, if IVIG is given within the first 10 days of the illness, it can decrease the risk of developing coronary changes from 25 percent to less than five percent.4
Even so, children who have had heart complications as a result of KD have an increased risk of developing cardiovascular complications later in life, including conditions such as heart attacks and heart disease.14 New research shows that children with KD remain at an increased risk for cardiovascular events more than 10 years after hospitalization for their condition, highlighting the need for long-term heart disease surveillance and risk reduction strategies.
One study in Ontario, Canada, was conducted to determine the risk and timing of long-term cardiovascular events and death among KD survivors. In the study, the researchers identified all children up to 18 years of age who survived hospitalization for KD in Ontario between 1995 and 2018 using health administrative databases. They included only the first eligible hospitalization, excluding children who were previously diagnosed with KD, as well as non-residents of Ontario. They matched each KD case to 100 non-exposed control cases by age, sex and year. After following these patients until death or March 2019, or up to 24 years old, they determined the rates of cardiovascular events, major adverse cardiac events (such as heart attack or stroke) and death, comparing children who had KD with those who were not exposed to the disease. Specifically, they looked at four time periods after hospital discharge: zero to one year, one to five years, five to 10 years and more than 10 years.
The researchers found that, among 4,597 KD survivors, 746 or 16.2 percent experienced cardiovascular events compared with 5.2 percent of children without the disease. They also found that 79 or 1.7 percent experienced major adverse cardiac events compared to 0.7 percent of children without the disease, and nine died during the median 11-year follow-up period. The most frequent cardiovascular events experienced by KD survivors were ischemic heart disease, arrhythmias, high blood pressure and peripheral vascular disease. KD survivors were at higher risk of heart problems compared to patients who did not have the disease, and they experienced cardiovascular events sooner. Their risk was highest in the first year after they were discharged from the hospital. They were also at higher risk of heart surgery like coronary artery bypass grafting. However, their risk of death during follow-up was lower than non-exposed patients.15
Dispelling the Myths Now
Research continues in an effort to uncover the causes of KD. According to a paper published in Clinical and Experimental Pediatrics in 2022, “the leading theory for KD’s pathogenesis is that an unknown infectious agent activates the immune system of a genetically susceptible child.” According to the paper’s author, several findings support this theory, including that KD occurs most commonly in the cold winter season when an unknown infectious agent activates the immune system of a genetically susceptible child; the significant overlap of clinical symptoms of KD and other infectious substances, particularly scarlet fever, newly described MIS-C and adenovirus; and reports that demonstrated the incidence of KD significantly decreased during the COVID-19 pandemic with the implementation of mask wearing and social distancing measures.16
In a 2024 paper published in The Journal of Clinical Investigation, the author notes that model predictions indicate that by 2030, one in every 1,600 adults will have experienced KD in the United States. He also points out that “once the etiology is solved, specific diagnostic tests will follow and epidemiology based on confirmed diagnoses will be possible.”17 In the meantime, with a better awareness of what is believed to be a rare disease but in fact is not, as well as dispelling the many myths concerning it, it is hoped that diagnoses of KD will be swifter to curtail the development of acute KD in children that leads to long-term health consequences.
References
1. UC San Diego School of Medicine Department of Pediatrics. Kawasaki Disease. Accessed at pediatrics.ucsd.edu/research/centers/kawasaki-disease/about/history.html.
2. Burns, JC, Kushner, H, Bastian, JF, et al. Kawasaki Disease: A Brief History. Pediatrics, 2000 Aug;106(2):E27. Accessed at pubmed.ncbi.nlm.nih.gov/10920183.
3. Uehara, R, and Belay, ED. Epidemiology of Kawasaki Disease in Asia, Europe, and the United States. Journal of Epidemiology, v.22(2); 2012. Accessed at www.ncbi.nlm.nih.gov/pmc/articles/PMC3798585.
4. Cincinnati Children’s. Kawasaki Disease in Children. Accessed at www.cincinnatichildrens.org/health/k/kawasaki.
5. Butters, C, Curtis, N, and Burgner, DP. Kawasaki Disease Fact Check: Myths, Misconceptions, and Mysteries. Journal of Pediatrics and Child Health, Aug. 8, 2020. Accessed at onlinelibrary.wiley.com/doi/10.1111/jpc.15101.
6. Barnes, BT. Kawasaki Disease. Johns Hopkins Medicine. Accessed at www.hopkinsmedicine.org/health/conditions-and-diseases/kawasaki-disease.
7. Ae, R, Makino, N, Kuwabara, M, et al. Incidence of Kawasaki Disease Before and After the COVID-19 Pandemic in Japan. JAMA Pediatrics, 2022 Dec; 176(12): 1217–1224. Accessed at www.ncbi.nlm.nih.gov/pmc/articles/PMC9577881.
8. Leduc, C. Kawasaki Disease: 10 Frequently Asked Questions, Answered. Ameripharma, May 1, 2024. Accessed at ameripharmaspecialty.com/other-health-conditions/kawasakidisease-10-frequently-asked-questions-answered.
9. Mayo Clinic. Kawasaki Disease Symptoms and Causes. Accessed at www.mayoclinic.org/diseases-conditions/kawasaki-disease/symptoms-causes/syc-20354598.
10. Mayo Clinic. KawasakiDiseaseDiagnosis and Treatment. Accessed at www.mayoclinic.org/diseases-conditions/kawasaki-disease/diagnosis-treatment/drc-20354603.
11. Ig Governance. Kawasaki Disease (Mucocutaneous Lymph Node Syndrome). Accessed at www.criteria.blood.gov.au/MedicalCondition/View/2692.
12. Halpern, L. High-Dose IVIG with Glucocorticoids Shown to Benefit Patients with Kawasaki Disease at High Risk for IVIG Resistance. Pharmacy Times,Oct. 15, 2024. Accessed at www.pharmacytimes.com/view/high-dose-ivig-with-glucocorticoids-shown-to-benefitpatients-with-kawasaki-disease-at-high-risk-for-ivig-resistance.
13. Wang, J, Chen, H, Shi, H, et al. Effect of Different Doses of Aspirin on the Prognosis of Kawasaki Disease. Pediatric Rheumatology, 2020, 18(48). Accessed at ped-rheum.biomedcentral.com/articles/10.1186/s12969-020-00432-x.
14. United Kingdom National Health Service. Kawasaki Disease Complications. Accessed at www.nhs.uk/conditions/kawasakidisease/complications.
15. Children with Kawasaki Disease at Increased Risk for Cardiovascular Events 10 Years Later. NewsMedical, Nov. 6, 2020. Accessed at www.news-medical.net/news/20201106/Children-with-Kawasaki-Diseaseat-increased-risk-for-cardiovascular-events-10-years-later.aspx.
16. Woo, H-O. Recent Research Trends in Kawasaki DiseaseRelated Infection. Clinical and Experimental Pediatrics, 2022 Jul 22;65(11):538–539. Accessed at pmc.ncbi.nlm.nih.gov/articles/PMC9650356.
17. Burns, JC. The Etiologies of Kawasaki Disease. The Journal of Clinical Investigation, March 1, 2024. Accessed at www.jci.org/articles/view/176938.
