In the R&D Pipeline: New Agents to Prevent and Treat Hereditary Angioedema Attacks
- By Keith Berman, MPH, MBA
TISSUE EDEMA is a normal component of the acute inflammatory response to localized injury caused by invading pathogens, trauma, toxins, heat or other causes. This protective tissue swelling phenomenon is regulated in part by a protease inhibitor called complement 1 esterase inhibitor (C1-INH).
But individuals affected with hereditary angioedema (HAE) experience recurrent attacks of localized subcutaneous and mucosal swelling, the results of a deficient level of functional C1-INH (type I disease), or a normal level of a dysfunctional C1-INH protein (type II disease). (A third category of HAE is characterized by normal C1-INH levels and function, but with angioedema symptoms similar to those affecting people with types I and II HAE.) Roughly one in 50,000 people are living with this rare autosomal dominant disorder, which results from more than 200 known mutations affecting the SERPING1 gene that encodes C1-INH.
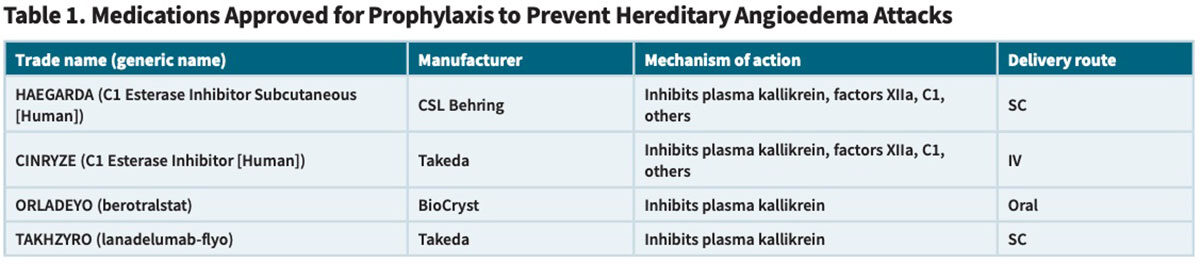
These acute and often painful attacks of soft tissue swelling typically involve the face, mouth, tongue, oropharynx, hands, feet, genitals or intestinal tract. While the frequency, severity and localization of these angioedema attacks varies widely from one individual to the next, at least 50 percent of untreated patients eventually experience laryngeal attacks, the worst of which can obstruct the upper airway and result in fatal asphyxiation. Through multiple complex pathways (Figure), C1-INH works to modulate the extent of increased microvascular permeability and edema that naturally occurs during an inflammatory reaction (Figure). Specifically, C1-INH is the only known inhibitor that acts to regulate the conversion of prekallikrein to kallikrein. In persons with HAE, C1-INH deficiency or dysfunction leads to excessive kallikrein production, which in turn causes uncontrolled cleavage of high molecular-weight kininogen (HMWK) to yield supra-normal levels of the potent vasodilator bradykinin. This excessive bradykinin binds to the bradykinin B2 receptor on vascular endothelial cells, further increasing vascular permeability that results in excessive localized swelling, inflammation and pain associated with HAE attacks.
Today’s Options to Treat and Prevent HAE Attacks
The management of HAE attacks is addressed therapeutically in two ways: 1) prophylaxis with medications to prevent HAE attacks from happening in the first place, or at least reduce their number and severity, and 2) treatment of HAE attacks, including breakthrough attacks in patients on drug prophylaxis, with medications that can limit their severity and shorten the time to symptomatic relief and resolution.
Approved Prophylaxis Options
Prior to the approval of the first human C1-INH concentrates 15 years ago, HAE therapy was largely limited to long-term prophylaxis with antifibrinolytics, which were not reliably effective,1 and with attenuated androgens, whose chronic administration was frequently associated with hepatotoxicity, virilization and other intolerable side effects.2
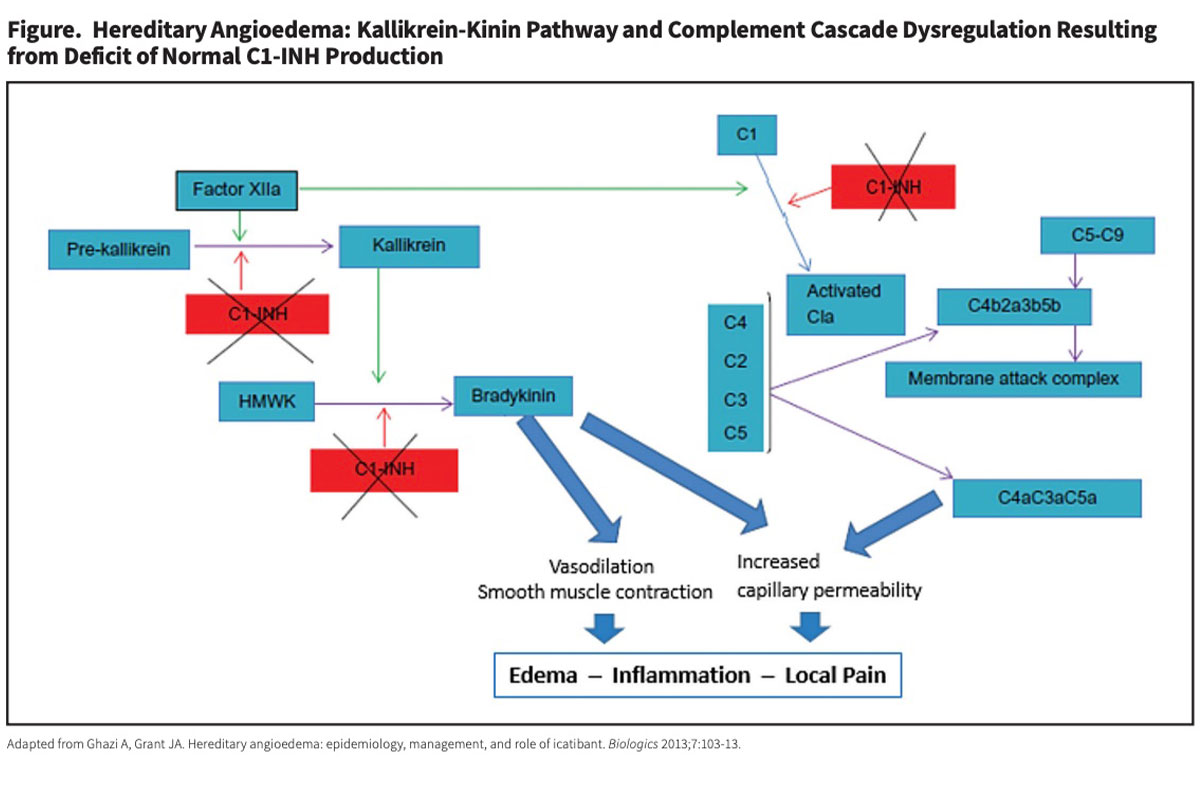
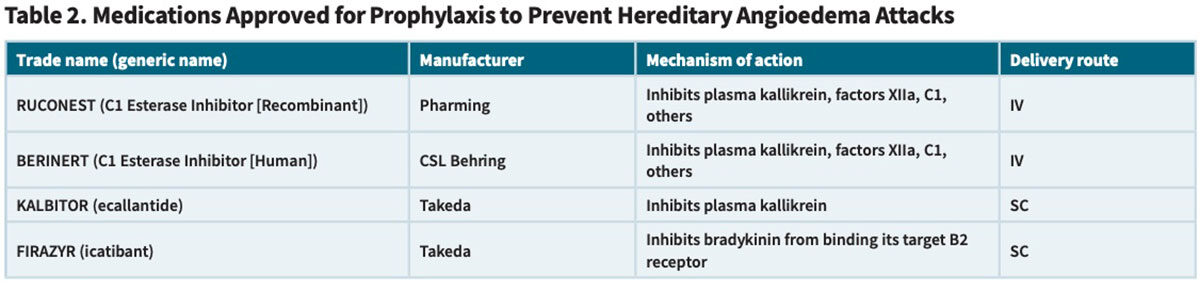
But the approvals of eight new HAE treatments over the last 15 years have transformed the management of adults and children with HAE. Very broadly, these agents can be classified in two ways: 1) replacement C1-INH therapies versus synthetic inhibitors that interfere with the kallikrein-kinin pathway, and 2) approved use for prophylaxis to prevent HAE attacks versus treatment of acute HAE attacks (Tables 1 and 2).
CINRYZE (C1 esterase inhibitor [human]). In 2008, Takeda’s CINRYZE became the first human C1-INH product approved by the U.S. Food and Drug Administration (FDA) for routine prophylaxis to prevent HAE attacks. Approval was based on results from a study in 22 subjects randomly assigned to receive intravenous (IV) injections of saline placebo or 1,000 units of CINRYZE every three to four days for 12 weeks, then crossed over to the alternative treatment for a second 12-week evaluation period.3 Mean attack rates during the two 12-week crossover periods were 6.26 and 12.73 for the C1-INH and placebo treatments, respectively. The differences in mean attack severity on C1-INH prophylaxis versus placebo (1.3 versus 1.9 on a 3-point scale) and total duration of attacks (2.1 versus 3.4 days) were also highly significant.
While all these outcomes are clinically important and clearly justify the use of C1-INH, the substantial frequency of breakthrough HAE attacks reveals that simply replacing C1-INH does not provide complete protection. Further boosting the dose to try to reduce breakthrough attacks is also problematic, as IV-administered C1-INH has been associated with reports of serious arterial and venous thromboembolic events (TEEs).
HAEGARDA (C1 esterase inhibitor [human]). Almost a decade later in 2017, CSL Behring introduced HAEGARDA, its own human plasma-derived C1-INH product for routine prophylaxis to prevent HAE attacks in patients 6 years of age and older. But unlike delivery of CINRYZE by IV infusion, HAEGARDA is administered subcutaneously (SC). Because C1-INH has a half-life of just 20 hours, twice-weekly SC delivery of a much higher dose results in a smoothing of the pronounced “sawtooth” pharmacokinetic profile, with higher and better sustained trough levels than occurs with twice-weekly IV administration of CINRYZE.4 Not surprisingly, a recent study showed that, in patients who received twice-weekly intravenous C1-INH prophylaxis, breakthrough angioedema attacks tended to occur shortly before the next scheduled infusion, when the circulating level of infused C1-INH reaches very low nadir. This problem is essentially eliminated with higher-dose SC administration of HAEGARDA.5
A prospective, randomized, double-blind, crossover, placebo-controlled Phase III trial of self-administered SC HAEGARDA yielded unprecedented positive results: At a 60 IU/kg dose, the time-normalized median number of attacks was reduced by 87 percent versus placebo (0.52 versus 4.03 attacks per month).6 Moreover, the average severity of HAE attacks was far milder in HAEGARDA-treated patients than those randomized to placebo; just nine percent of attacks were graded as “severe,” compared to 69 percent of attacks in the placebo group. Fully 40 percent of study subjects did not experience any attacks over the 16-week treatment period, as compared with no attack-free subjects in the placebo group.7
This improved protection of HAEGARDA against HAE attacks relative to IV-administered CINRYZE is largely attributable to the ability of its twice-weekly SC dosing with 60 IU/kg to restore and maintain the circulating level of C1-INH above 40 percent of normal, which has shown to be associated with reduced risk of HAE attacks.
On the strength of its superior performance, a more convenient SC administration route and lack of evidence of any causal association with TEEs, HAEGARDA has largely supplanted CINRYZE as the preferred plasmaderived C1-INH product for use as prophylactic therapy in HAE patients.8
TAKHZYRO (lanadelumab-flyo). For patients prescribed routine prophylaxis to prevent attacks, HAEGARDA now mainly competes with two recently licensed non-plasma-based therapies (Table 1). Approved in 2018 for prevention of HAE attacks in adult and pediatric patients ages 2 years and older, Takeda’s TAKHZYRO is a fully human monoclonal antibody that binds plasma kallikrein, inhibiting its ability to cleave HMWK and generate bradykinin.
Adult clinical trial subjects who self-administered a 300 mg SC dose of TAKHZYRO on a biweekly basis experienced 83 percent fewer moderate to severe HAE attacks, and 87 percent fewer attacks requiring on-demand treatment, than placebo group subjects. Also generally similar to Phase III study results with HAEGARDA, 77 percent of these subjects were attack-free during their steady-state day 70-182 treatment period, compared to just three percent of placebo group subjects.9
ORLADEYO (berotralstat). Approved in late 2020, BioCryst Pharmaceuticals’ ORLADEYO is the first and currently the only once-daily oral therapy indicated for prophylaxis to prevent HAE attacks in adult and adolescent patients. Like TAKHZYRO, ORLADEYO binds to plasma kallikrein, inhibiting its bradykinin-generating activity.
In a Phase III trial, the recommended 150 mg dose of berotralstat achieved a 44 percent mean reduction in the monthly HAE attack rate, somewhat lower than that attained with HAEGARDA or TAKHZYRO. No difference between groups was observed in the proportion of attack-free patients. Adverse reactions occurring at a notably higher rate in berotralstat group patients than in placebo group patients included abdominal pain, vomiting, diarrhea and back pain, but typically self-resolved and became less frequent over time.10
Approved On-Demand Treatment Options
More than a decade ago, two other C1-INH products also received FDA approval specifically for the treatment of acute HAE attacks: one purified from human donor plasma (CSL Behring’s BERINERT) and one produced through recombinant DNA technology and purified from the milk of transgenic rabbits (Pharming’s RUCONEST).
Also approved are two SC-administered, non-plasma-based drugs (Table 2) designed to treat HAE attacks by interfering with the kallikrein-bradykinin pathway: Takeda’s plasma kallikrein inhibitor KALBITOR (ecallantide) and its bradykinin B2 receptor antagonist FIRAZYR (icatibant). While FIRAZYR can be self-administered by the patient upon recognition of an HAE attack, KALBITOR must be administered by a healthcare professional because of a very small risk (<2 percent) of allergic or anaphylactic reaction, effectively prolonging the time to treatment.
Two key treatment goals with all of these agents are to 1) reduce the time elapsed to achieve a specified reduction from baseline symptoms (or attain greater reduction of symptoms at a specified posttreatment time point), and 2) reduce the time to complete resolution of symptoms. An additional endpoint of interest is the usage of additional rescue medication; less frequent need for rescue medication is another important indicator of the product’s effectiveness in shortening the time to symptom relief.
In 2021, the Hereditary Angioedema Association’s (HAEA) Medical Advisory Board updated its published guidelines that cite key HAE management principles and product-specific efficacy and safety information to help clinicians choose from the various on-demand and prophylaxis treatment options. The 11 specialists who authored these guidelines emphasize that much of the decision-making process should reflect the reality that “HAE has a highly variable clinical course with numerous presentations and symptoms.”11
HAE Therapies in the R&D Pipeline
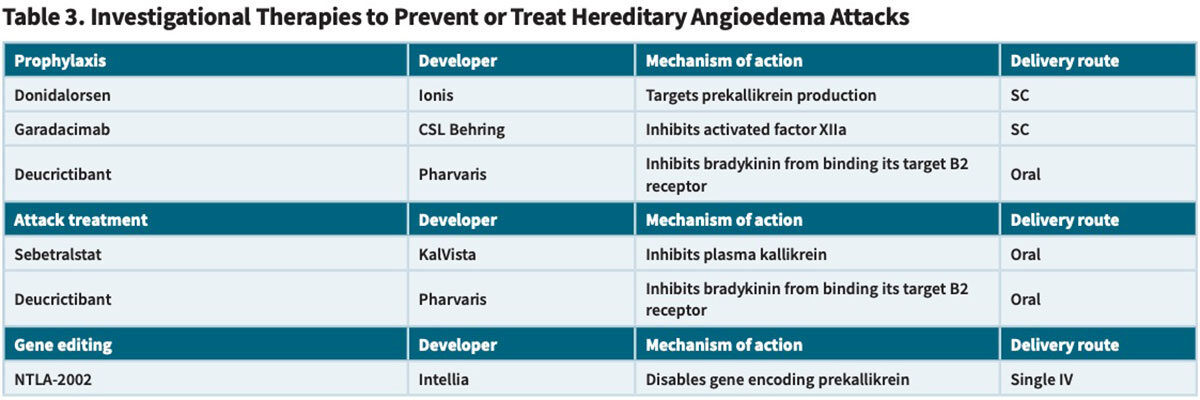
The fact that clinicians now have an impressive armamentarium of HAE management options has not dissuaded a number of companies from developing entirely new potential therapies to treat or prevent HAE attacks (Table 3), in the hope of pushing the envelope to demonstrate enhanced efficacy, tolerability and/or patient usage convenience.
Investigational Prophylaxis Therapies
Donidalorsen (Ionis Pharmaceuticals). This RNA ligand-conjugated antisense (LICA) agent is designed to precisely target and silence the production of prekallikrein, thereby interrupting the pathway that leads to HAE attacks. Ionis’ recently completed Phase III OASIS-HAE trial randomized subjects with HAE to a SC 80 mg dose of donidalorsen or placebo every four weeks (Q4W) or every eight weeks (Q8W), and found that the mean attack rate from week five to week 25 was 87 percent lower in the Q4W group and 60 percent lower in the Q8W group than in the placebo group.12
In a separate Phase II open-label extension study evaluating flexible dosing of donidalorsen over two years, five of the eight subjects who received a single SC injection every eight weeks remained attack-free, while the other three subjects were switched to receive the drug every four weeks.13 The mean monthly attack rate across all eight subjects declined from 0.28 attacks in year one to 0.04 attacks in year two. “At year two, donidalorsen Q8 was well-tolerated, had plasma prekallikrein levels similar to Q4W, and showed durable efficacy in HAE attack reduction, supporting the continued study of Q8W dosing,” the study investigators concluded.
These findings suggest that the dosing frequency of donidalorsen could potentially be individualized based on the requirement to achieve protective efficacy against HAE attacks. A significant subset of fortunate HAE patients could require a single SC injection as infrequently as every eight weeks to remain attack-free.
Garadacimab (CSL Behring). Also identified as CSL312, this novel, fullyhuman monoclonal antibody interferes with the cascade of events leading to edema formation by inhibiting activated factor XII (FXIIa). Subcutaneous dosing of garadacimab starts with a 400 mg loading dose, followed by monthly selfadministered doses.
According to Phase III VANGUARD study findings reported last year, the mean number of HAE attacks in adult and adolescent participants randomized to receive garadacimab for six months was 87 percent lower than in the placebo group.14 Both safety and tolerability appeared favorable, and FXIIa inhibition was not associated with an increased risk of bleeding or thromboembolic events. “These results underscore our belief that garadacimab has the potential to become a transformative first-in-class therapy for people living with HAE,” said CSL’s head of R&D and chief medical officer Bill Mezzanotte, MD, MPH.
Very encouragingly, garadacimab prophylaxis yielded greater than a 94 percent reduction in the number of attacks compared to the run-in period over a median exposure period of nearly 14 months. A remarkable 88 percent of patients were attack-free at the end of the 13- to 15-month open-label observation period. A biologics license application has been submitted to FDA, and CSL anticipates marketing approval sometime in the first half of 2025.15
Investigational On-Demand Treatment Therapies
Sebetralstat (KalVista Pharmaceuticals). Results from the placebo-controlled Phase III KONFIDENT trial involving 136 participants experiencing HAE attacks demonstrate sharp improvements in multiple outcome parameters for both those who received a single oral dose of this investigational plasma kallikrein inhibitor.16 The median time to the start of symptom relief was significantly faster with the 300 mg (1.61 hours) and 600 mg doses (1.79 hours) than placebo (6.72 hours), as was the time to reduction in attack severity (9.27, 7.75 and >12 hours, respectively). The percentage of attacks with complete resolution within 24 hours was also higher with sebetralstat treatment (42.5 percent, 49.5 percent and 27.4 percent, respectively).
If approved, sebetralstat would become the first orally self-administered treatment for HAE attacks. “Having a safe and effective oral, on-demand treatment for HAE attacks could be immensely valuable in addressing unmet needs and reducing the treatment burden associated with current injectable treatments,” said KONFIDENT trial lead investigator Marc Riedl, MD.
Deucrictibant (Pharvaris). This Dutch R&D-stage company has developed an oral small-molecule bradykinin B2 receptor antagonist that has generated positive data in both Phase II on-demand and prophylaxis studies in patients with HAE. A multinational Phase II on-demand treatment trial involving 34 participants demonstrated an 84.5 percent reduction in the overall monthly attack rate with a daily 40 mg dose.17 Even more impressive, the frequency of moderate and severe attacks was reduced by 92 percent; relative to 0.45 severe monthly attacks in the placebo group, there were no attacks in a total of 23 subjects who received either the 40 mg or a smaller 20 mg daily dose.
Earlier this year, Pharvaris initiated its Phase III, placebo-controlled cross-over RAPIDe-3 study to evaluate the efficacy and safety of an immediate-release capsule form of deucrictibant for the on-demand treatment of HAE attacks, including non-severe laryngeal attacks, in patients both using and not using long-term prophylactic medications.
Potentially Curative Gene Editing Therapy
Massachusetts-based Intellia Therapeutics has developed an investigational in vivo CRISPR-based gene editing therapy, dubbed NTLA2002, that in an ongoing Phase II study has achieved what the company describes as “durable elimination” of HAE attacks in HAE patients. NTLA-2002 is designed to disable the prekallikreinencoding KLKB1 gene in liver cells to prevent the downstream production of kallikrein, in turn “rebalancing” the HAE patient’s kallikrein-kinin pathway and reducing excessive bradykinin production.
At both 25 mg and 50 mg doses, a single infusion of NTLA-2002 in a total of 21 study subjects resulted in an 80 percent reduction in monthly angioedema attacks relative to six risk-matched placebo group subjects. Even more promising, four of 10 subjects dosed with 25 mg and eight of 11 subjects dosed with 50 mg have remained completely attack-free over the 16-week observation period — a complete response with no requirement for any subsequent treatment.18
“Phase II data continue to reinforce the potential of a single dose of NTLA-2002 to be a functional cure for patients with HAE,” investigators said in a presentation at this year’s American College of Allergy, Asthma & Immunology Annual Scientific Meeting in Boston.
Normalizing Life with HAE
HAEA’s Medical Advisory Board points out that “HAE is a chronic condition with tremendous variability in symptom quality, frequency and severity,” necessitating that “HAE management plans be individualized with treatment tailored to each patient’s medical needs, life circumstances and preferences, as well as tolerance of and response to specific medications.”11 This individualized approach, even for individuals with the most severe HAE clinical manifestations, has already allowed many to lead a normal life.
A number of promising new agents in the R&D pipeline are likely to be approved in the very near future, expanding an already impressive range of treatment options — while further increasing the complexity of the HAE management decision process.
But with each unique new therapy will also come the opportunity for clinicians to help more people living with HAE to finally free themselves from this frightening and disabling disease.
References
- Nzeako UC, Frigas E, Tremaine WJ. Hereditary angioedema: A broad review for clinicians. Arch Int Med 2001 Nov 12;161:2417-29.
- Cicardi M, Bergamaschini L, Tucci A, et al. Morphologic evaluation of the liver in hereditary angioedema patients on long-term treatment with androgen derivatives. J Allergy Clin Immunol 1983;72:294-8.
- Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. New Engl J Med 2010 Aug 5;363:513-22.
- Longhurst, HJ, Cicardi M, Craig T, et al. Prevention of hereditary angioedema attacks with a subcutaneous C1 inhibitor. New Engl J Med 2017;376:1131-40.
- Aygoren-Pursun E, Martinexz-Saguer I, Longhurst HJ, et al. C1 inhibitor for routine prophylaxis in patients with hereditary angioedema: interim results from a European Registry study. J Allergy Clin Immunol 2016;137:Ab251 (abstract).
- HAEGARDA C1 Esterase Inhibitor Subcutaneous (Human). Full prescribing information. CSL Behring. Revised January 2022. Accessed at labeling.cslbehring.com/PI/US/HAEGARDA/EN/HAEGARDA-Prescribing-Information.pdf.
- Longhurst H, Cicardi M, Craig T, et al. Prevention of hereditary angioedema attacks with a subcutaneous C1 inhibitor. New Engl J Med 2017 Mar 23;376(12):1131-40.
- Marketing Research Bureau, Inc. The Plasma Proteins Market in the United States (2014-2023 report editions).
- Benerji A, Riedl MA, Bernstein JA, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA Nov 27;320(20):2108-2121.
- Zuraw B, Lumry WR, Johnston DT, et al. Oral once-daily berotralstat for the prevention of hereditary angioedema attacks: a randomized, double-blind, placebo-controlled phase 3 trial. J Allergy Clin Immunol 2021;148:164-172.
- Busse PJ, Christiansen S, Riedl MA, et al. US HAEA Medical Advisory Board 2020 Guidelines for the Management of Hereditary Angioedema. J Allergy Clin Immunol Pract 2021;9:132-50.
- Riedl MA, Tachdjian R, Lumry W, et al. Efficacy and safety of donidalorsen for hereditary angioedema. New Engl J Med 2024 Jul 4;391(1):21-31.
- Manning M, Bordone L, Newman K, et al. The impact of donidalorsen taken every 8 weeks in patients with hereditary angioedema: Two-year update. J Allergy Clin Immunol 2024 Feb;153(2 Suppl):AB2.
- Craig TJ, Reshef A, Li HH, et al. Efficacy and safety of garadacimab, a factor Xlla inhibitor for hereditary angioedema prevention (VANGUARD): a global, multicentre, randomized, double-blind, placebo-controlled, phase 3 trial. Lancet 2023 Apr 1;401(10382):1079-1090.
- CSL R&D Investor Briefing. October 22, 2024. Accessed at investors.csl/com/pdf/15cfdb5c-f8e2-4d90-8243-1253071853af/Research-and-Development-Investor-Briefing.pdf.
- Riedl MA, Farkas H, et al. Oral sebetralstat for on-demand treatment of hereditary angioedema attacks. N Engl J Med 2024 Jul 4;391(1):32-43.
- Wedner HJ, Anderson J, Chapdelaine H, et al. CHAPTER-1 Phase 2 trial of oral bradykinin B2 receptor antagonist deucrictibant for herditary angioedema prophylaxis. ACAAI Annual Scientific Meeting. October 24-28, 2024. Accessed at ir.pharvaris.com/static-files/06828095-81aa-4761-8050-6659e9f6515f.
- Cohn DM, Gurugama P, Magerl M, et al. Results from a Phase 2, randomized, placebo-controlled trial of CRISPR-based therapy NTLA-2002 for hereditary angioedema. ACAAI Annual Scientific Meeting. October 24-28, 2024. Accessed at www.intelliatx.com/wp-content/uploads/Ph2-Update_ACAAI-2024_Oral_21Oct24a_vF.pdf.