Human Albumin as Drug Therapy for Decompensated Cirrhosis: A New Lifesaving Role for an Old Player?
- By Keith Berman, MPH, MBA
“The mechanism behind current usage of albumin is that of volume expansion. However, many hepatologists believe albumin has other medicinal properties.”
— The ATTIRE Trial Investigators22
WHEN AN ACTOR is widely known for playing just one kind of part, we say he or she is “type-cast.” Since it was first purified and administered to severely burned and bleeding U.S. soldiers during World War II, the clinical role for human albumin has unquestionably been type-cast as well. As albumin comprises roughly half of total plasma protein content and accounts for 75 percent of plasma oncotic pressure, it was quite natural to assume maintenance of circulating plasma volume is the primary role of this relatively small 66 kilodalton protein.
And so it is that, for more than seven decades, albumin has been relegated to a supporting role as a simple plasma volume expander whose oncotic properties are needed in clinical situations where use or continued use of crystalloids is contraindicated. But, recent in vitro and small animal studies, and now surprising new findings from clinical studies evaluating 20 percent albumin in patients with decompensated cirrhosis, point to therapeutic mechanisms beyond simple maintenance of circulating blood volume. A growing body of evidence suggests known pharmacologic properties of human albumin may contribute to reducing the risk of a range of cirrhosis complications, including bacterial sepsis, irreversible renal injury and death.
Study Findings Spur Interest in Albumin Functionality
Interest in the physiologic role of circulating albumin began in earnest after publication of a landmark 1999 clinical study evaluating exogenous albumin as a blood volume expander for cirrhotic patients with spontaneous bacterial peritonitis (SBP), with the goal of minimizing renal impairment thought to be due to an infection-mediated drop in effective arterial volume.1 Spanish investigators randomized 126 patients to receive an intravenous antibiotic (cefotaxime) or antibiotic plus 1.5 gram/kg albumin administered at the time of diagnosis, followed by 1 gram/kg on day three of hospitalization. Both treatment groups experienced similarly high infection resolution rates (94 percent and 98 percent). But, the rate of nonreversible renal impairment (hepatorenal syndrome) was three-fold higher in the cefotaximeonly group than the cefotaxime-plusalbumin group (33 percent vs. 10 percent, p=0.001), as was in-hospital mortality (29 percent vs. 10 percent, p=0.01).
This single study transformed the management of cirrhosis with SBP; albumin administration at diagnosis and on day three is now the standard of care in qualifying patients.2 While prevention of circulatory dysfunction was considered the most likely explanation for how just two doses of exogenous albumin so sharply reduced rates both of hepatorenal syndrome and in-hospital mortality, the investigators noted “the possibility that the beneficial effects of albumin involve mechanisms other than those related to plasma expansion cannot be ruled out.”
Protein chemistry research over the last two decades has vastly expanded our understanding of the physiologic actions of human albumin. Largely through its extraordinary ligand-binding properties, albumin mediates a diverse range of important functions. In particular, albumin:
- Accounts for most of the antioxidant capacity of human serum via scavenging and limiting production of reactive oxygen species (ROS), binding inactivating mediators of oxidant damage (e.g., Cu2+ and Fe3+) and binding and delivering antioxidant molecules;3
- Detoxifies circulating liver metabolites, including bilirubin, and transporting them to disposal sites, including the liver for eventual excretion in bile;4
- Binds and transports fatty acids essential for energy metabolism and membrane synthesis;5
- Serves as the primary reservoir for nitric oxide (NO), which acts as a mediator of vasodilation, platelet aggregation and superoxide production and removal; and
- Stimulates proliferation and maintains integrity and function of proximal renal tubular cells.6
Albumin, Prostaglandin E2 and Infection Risk
But a decade ago, United Kingdom (UK) and German collaborators showed albumin functionality declines (assessed by measuring affinity of albumin fatty acid binding sites) with increasing liver disease severity, likely due to accumulation of toxins, drugs and other ligands or to permanent derangements of the protein itself. Further, worsening albumin function was found to be associated with increased mortality.7 The study authors speculated the diminished ability of circulating albumin to prevent oxidative stress damage might account for further decompensation. This might well turn out to be a contributing factor, but more recent work definitively points to another connection between impaired albumin functionality and mortality: increased risk of severe bacterial infection.
Patients with decompensated cirrhosis are highly prone to bacterial infection, which is either present or acquired during 25 percent to 30 percent of hospitalizations.8 Bacterial infection is also a major cause of decompensation and hospitalization in persons with cirrhosis. These infections tend to progress to sepsis or severe sepsis, resulting in a four-fold increase in the probability of death relative to noncirrhotic hospitalized patients with infection.9 Of cirrhosis patients who develop both infection and organ dysfunction, between 60 percent and 95 percent will die.10
In a 2014 report, a team of UK investigators observed circulating levels of a cyclooxygenase (COX)-derived lipid mediator called prostaglandin E2 (PGE2) are highly elevated in patients with decompensated cirrhosis — more than seven times as high as in healthy volunteers.11 PGE2 has broad immunosuppressive effects that increase susceptibility to bacterial infection through several pathways, including blunting of antimicrobial helper T cell activity, interference with immune cell trafficking into tissue compartments and suppression of phagocytosis.12,13 In both in vitro and in vivo models, they discovered plasma from decompensated cirrhosis patients suppressed pro-inflammatory cytokine secretion and bacterial killing in a PGE2-dependent manner; these effects were not seen with plasma from patients with stable cirrhosis who had lower circulating PGE2 levels.
Why is this important? Human albumin normally acts as a “sink” that avidly binds PGE2 and catalyzes its inactivation. But endogenous serum albumin levels are low in acutely decompensated cirrhosis patients due to impaired albumin synthetic function. On top of this, albumin’s PGE2–binding capacity is markedly impaired in decompensated patients.14,15
As predicted, administration of commercial human albumin solution prepared from healthy donors to these patients reduced their plasma PGE2 level and reversed the immunosuppressive properties of their plasma on repeat testing.
In this one seminal study, the UK investigators made three important discoveries:
1) Sharply elevated PGE2 is the underlying cause of the immunosuppression that accounts for increased infection risk in patients with decompensated cirrhosis;
2) The elevated PGE2 in these patients results from the characteristic combination of hypoalbuminemia and impaired albumin binding function; and
3) Infusion of exogenous commercial albumin solution reduces PGE2 levels and reverses its immunosuppressive activity.
In a new 2018 report,16 members of this same research team showed that, in patients with acute decompensation and acute-on-chronic liver failure, administration of 20 percent commercial human albumin to raise the serum albumin level to above 3 g/dL was again able to reverse plasma-mediated immunosuppression through binding and inactivation of PGE2. They proposed that albumin, administered to target a serum albumin greater than 3 g/dL, be repurposed as “an immune-restorative drug” in hospitalized patients with decompensated cirrhosis. The immediate goal would be to augment treatment of infection and prevent nosocomial infection, with the potential to reduce mortality, ICU admissions, hospital stays and antibiotic use.
Long-Term Albumin Use for Cirrhosis with Ascites
Meanwhile, two other European research teams independently advanced clinical trials intended to learn whether human albumin therapy administered on a long-term basis could influence the grim prognosis for cirrhotic patients who develop ascites; about 15 percent of these individuals die within one year, and nearly 50 percent succumb within five years.17
Thirty-three Italian hospitals participating in the investigator-initiated ANSWER study randomized 440 patients with cirrhosis and uncomplicated ascites to receive either standard medical treatment (SMT) comprising anti-aldosteronic drugs and furosemide, or SMT plus 40 grams of human albumin twice weekly for two weeks, followed by 40 grams weekly for up to 18 months.18
By Kaplan-Meier estimates, overall 18-month survival was significantly higher in the SMT plus albumin group than in the SMT group (77 percent vs. 66 percent; p=0.028), translating into a 38 percent reduction in the mortality hazard ratio (0.62, 95 percent CI 0.40-0.95). Stated in real-world terms, treating just seven patients with albumin would be expected to prevent one death at 18-month followup.19 With an incremental cost of less than $25,000 per quality-adjusted life year (QALY), long-term albumin treatment appeared to be cost-effective as well.
In their report published last June, the ANSWER study investigators concluded “long-term human albumin administration prolongs overall survival and might act as a disease-modifying treatment in patients with decompensated cirrhosis.” This is the first prospective trial to document a clear survival benefit of routine long-term albumin infusions in patients with cirrhosis and ascites.
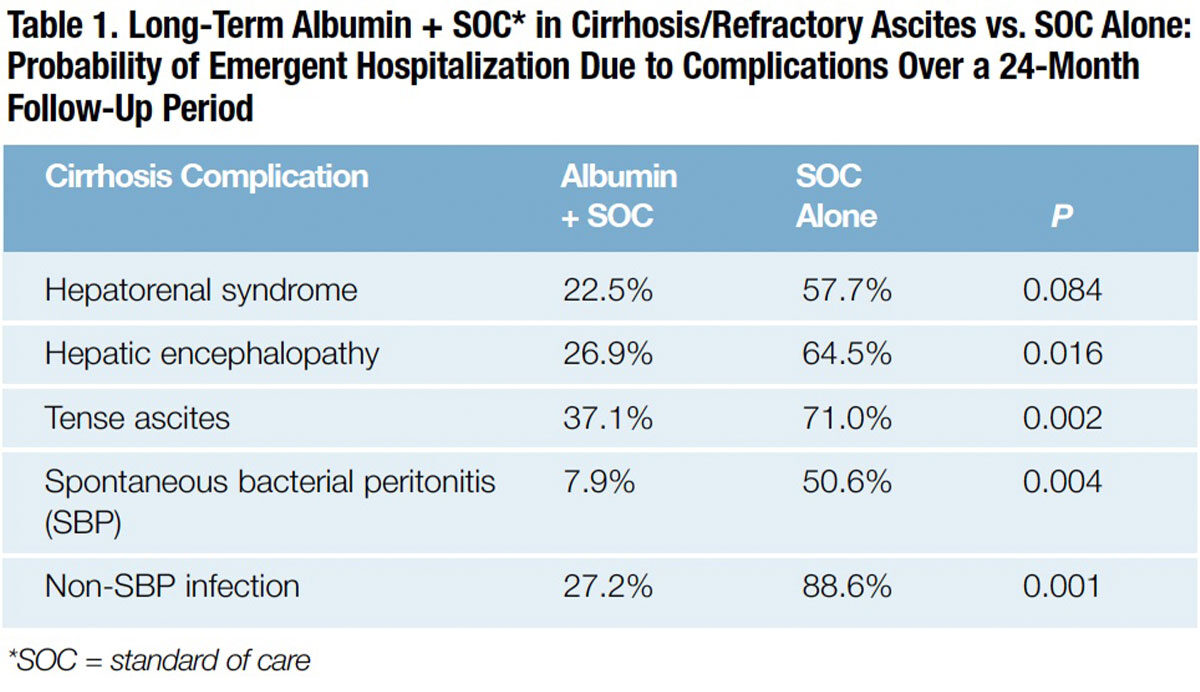
Just three months later in a separate single-center trial in Italy, investigators nonrandomly assigned 70 consecutively enrolled patients with cirrhosis and ascites refractory to standard of care (SOC) plus long-term albumin treatment (20 grams twice weekly), or SOC alone.20 The expected two-year survival in this more severely ill population, whose ascites fail to resolve with low-sodium diet and diuretic drugs, is just 30 percent.21 Patients received SOC when large-volume paracentesis was needed, and all patients were dosed with 6 grams to 8 grams of albumin per liter of ascites removed.
The cumulative incidence of 24-month mortality was significantly lower in the group treated with albumin and SMT than SOC alone (41.6 percent vs. 65.5 percent; p=0.032). Remarkably, compared to patients in the SOC group, patients with long-term albumin administration had far lower rates of emergent hospitalization for complications of cirrhosis, including hepatorenal syndrome, hepatic encephalopathy, tense ascites, SBP and non-SBP infections (Table 1). The study authors concluded that, in patients with cirrhosis and refractory ascites, long-term treatment with human albumin “significantly improved survival and reduced inpatient hospitalization.”
Now in Progress: Definitive Clinical Trials
While results of these two “pragmatic” clinical studies strongly suggest long-term administration of albumin to patients with decompensated cirrhosis can improve survival and reduce serious complications requiring hospitalization, these studies were not designed to provide a definitive answer. Most clinicians will require no less before they are willing to consider albumin as a therapeutic modality to be routinely administered, like a drug, on an extended or long-term basis.
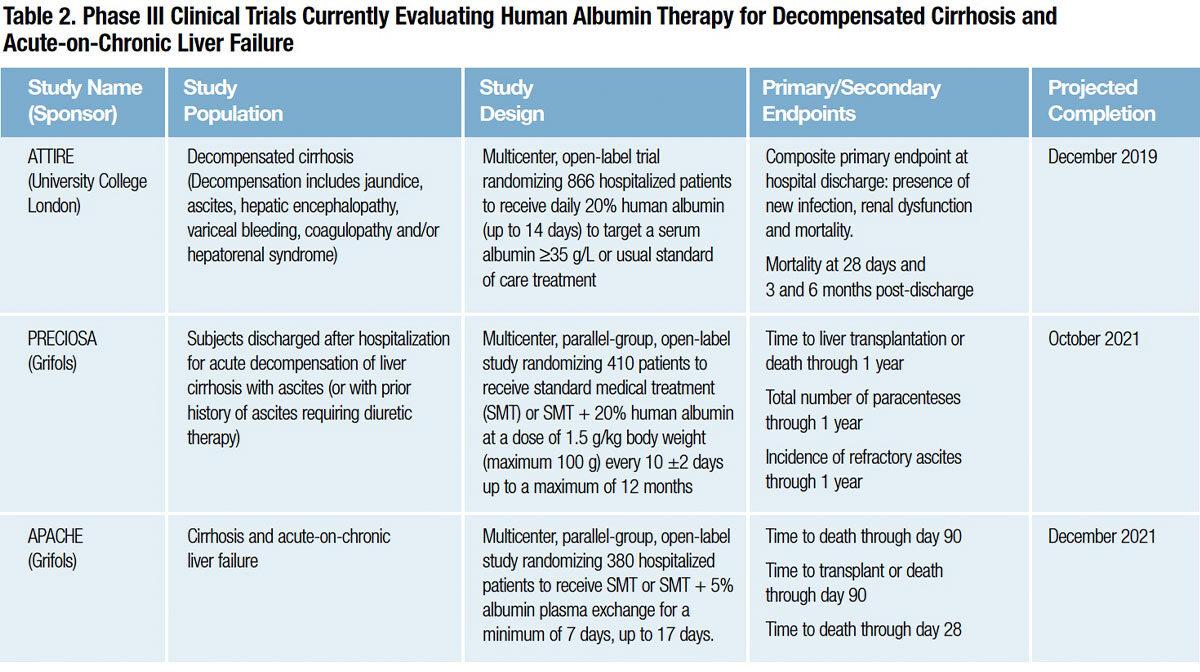
Several Phase III clinical trials now in progress should conclusively answer whether in-hospital or long-term human albumin therapy can meaningfully improve the prognosis for patients with decompensated cirrhosis or acute-on-chronic liver failure (Table 2):
- The ATTIRE (Albumin To prevenT Infection in chronic liveR failurE) study is randomizing patients admitted to the hospital with decompensated cirrhosis and a serum albumin level of less than 35 grams/L to receive standard medical care or daily 20 percent human albumin infusions to raise and maintain levels above 30 grams/L. The composite primary endpoint at hospital discharge is 1) presence of new infection, 2) renal dysfunction and 3) mortality.22
- The PRECIOSA (Prevention of Mortality with Long-Term Administration of Human Albumin in Subjects with Decompensated Cirrhosis) study is testing whether long-term administration of 20 percent human albumin can reduce mortality in patients with ascites following discharge from the hospital.23
-
The APACHE (Acute-on-Chronic Liver Failure Plasma Exchange) study is evaluating plasma exchange with 5 percent human albumin replacement to learn whether it can prolong short-term survival in patients with acute-on-chronic liver failure at very high risk of in-hospital mortality.24
Additionally, the Phase II HEAL (Hepatic Encephalopathy and Albumin) study is currently assessing whether up to five weekly infusions of 1.5 gram/kg of 25 percent human albumin can reduce cognitive impairment in patients with hepatic encephalopathy, a highly prevalent complication of cirrhosis that is both an independent risk factor for mortality and the leading cause of cirrhosis-related hospital readmissions.25
As with the other studies evaluating albumin for decompensated cirrhosis or liver failure, the therapeutic principle is entirely unrelated to albumin’s oncotic function. Inflammation, endotoxemia, oxidative stress and endothelial dysfunction all play an important role in the pathogenesis of hepatic encephalopathy. The capacity of already depressed levels of circulating albumin to bind and remove the metabolites that cause these problems is impaired in advanced cirrhosis. Infusions of normal human albumin purified from plasma of healthy donors serve to restore critical antioxidant and other functions that endogenous albumin is unable to provide.
A New Starring Role for Human Albumin?
For three-quarters of a century, hospital pharmacies and blood banks have dispensed concentrated 25 percent human albumin solutions for very short-term use to essentially restore circulating volume or fluid balance between the intravascular and extravascular compartments. Twenty years after the surprising discovery that albumin can dramatically reduce renal impairment and death in patients with cirrhosis and SBP, two new clinical studies provide similar evidence of these benefits — and more — with administration of concentrated albumin in patients with cirrhosis and uncomplicated or refractory ascites.18,20 Still other new findings suggest albumin-mediated immune-restorative and possibly other ligand binding-related functionalities may be at play.16
With at least three pivotal studies now in progress in critically ill cirrhosis patients, we will soon know whether medical science had simply overlooked some extraordinary pharmacologic talents of a plasma product it had long ago type-cast as a humble blood volume expander. A new second act for human albumin — arguably the single most versatile performer in the human proteome — may just be opening.
References
- Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med 1999;341(6):403-9.
- Runyan BA. Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. American Association for the Study of Liver Disease (AASLD). Accessed 1/24/2019 at www.aasld.org/publications/ascites-due-cirrhosis-management.
- Quinlan GJ, Mumby S, Martin GS, et al. Albumin influences total plasma antioxidant capacity favorably in patients with acute lung injury. Crit Care Med 2004 Mar;32(3):755-9.
- Wang X, Chowdhury JR, Chowdhury NR. Bilirubin metabolism: Applied physiology. Curr Paediatr 2006 Feb;16(1):70-4.
- Van der Vusse GJ. Albumin as fatty acid transporter. Drug Metab Pharmacokinet 29;24(4):300-7.
- Lee YJ and Han HJ. Albumin-stimulated DNA synthesis is mediated by Ca2+/PKC as well as EGF receptor-dependent p44/2 and NF-kappaB signal pathways in renal proximal tubule cells. Am J Physiol Renal Physiol 2008 Mar;294(3):F534-41.
- Jalan R, Schnurr K, Mookerjee RP, et al. Alterations in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. Hepatology 2009 Aug;50(2):555-64.
- Fernández J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology 2012;55:1551-61.
- Arvaniti V, D-Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010;139:1246-56.
- O’Brien AJ, Welch CA, Singer M, et al. Prevalence and outcome of cirrhosis patients admitted to UK intensive care: a comparison against dialysis-dependent chronic renal failure patients. Intensive Care Med 012;38:991-1000.
- O’Brien AJ, Fullerton JN, Massey A, et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med 2014 May;20:518-23.
- Ibid.
- Yang J, Petersen CE, Ha CE, et al. Structural insights into human serum albumin-mediated prostaglandin catalysis. Protein Sci 2002;11:538-45.
- Klammt S, Mitzner S, Stange J, et al. Albumin-binding function is reduced in patients with decompensated cirrhosis and correlates inversely with severity of liver disease assessed by model for end-stage liver disease. Eur J Gastroenterol Hepatol 2007;19:257-63.
- Jalan R, Schnurr K, Mokerjee RP, et al. Alterations in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. Hepatology 2009;50:555-64.
- China L, Maini A, Skene SS, et al. Albumin counteracts immunesuppressive effects of lipid mediators in patients with advanced liver disease. Clin Gastroenterol Hepatol 2018;16:738-47.
- Planas R, Montoliu S, Balleste B, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol 2006;4:1385-94.
- Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet 2018 Jun 16;391(10138):2417-29.
- Zaman A. Long-term albumin infusion might improve survival in decompensated cirrhosis. NEJM Journal Watch 2018 Jun 8. Accessed 2/2/2019 at www.jwatch.org/na46899/2018/06/08/long-termalbumin-infusion-might-improve-survival.
- Di Pascoli M, Fasolato S, Piano S, et al. Long-term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int 2019 Jan;39(1):98-105.
- Ginès P, Cárdenas A, Arroyo V, et al. Management of cirrhosis and ascites. N Engl J Med 2004;350:1646-54.
- China L, Skene SS, Bennett K, et al. Albumin To prevenT Infection in chronic liveR failurE: study protocol for an interventional randomised controlled trial. BMJ Open 2018;8:e023754.
- ClinicalTrials.gov. Accessed 2/18/2019 at clinicaltrials.gov/ct2/show/NCT03451292?term=PRECIOSA&cond=cirrhosis&rank=1.
- ClinicalTrials.gov. Accessed 2/18/2019 at clinicaltrials.gov/ct2/show/NCT03702920?term=Apache&cond=Liver+Failure%2C+Acute+on+Chronic&rank=1.
- ClinicalTrials.gov. Accessed 2/18/2019 at clinicaltrials.gov/ct2/show/NCT03585257?term=albumin&recrs=ab&cond=cirrhosis& draw=2&rank=11.
