CMS Proposed Payment Rules for 2025
- By Bonnie Kirschenbaum, MS, FASHP, FCSHP
HEALTHCARE can be delivered in myriad different sites of care, and each of these has a Centers for Medicare and Medicaid Services (CMS) rule set that governs conditions of participation and the payment for goods and services. These rules and rates are updated and amended every year.
In addition to proposed payment rate changes, this year’s proposed rules also include implementing policies that address health disparities, expand access to behavioral healthcare, improve transparency and promote safe, effective, patient-centered care.1 Here are key highlights of proposed changes for the upcoming year.
New Rules for Inpatient and Long-Term Care Hospitals
Effective Oct. 1, 2024:
• 2.9 percent payment rate increase for hospitals that are meaningful users of electronic health records and submit quality measure data
• Separate inpatient prospective payment system payment for small, independent hospitals to establish and maintain access to essential medicines
• An initiative to address homelessness, which may lead to higher payouts for hospitals treating patients with housing needs
• Requirement for long-term care hospitals to report social needs such as housing and food insecurity
Further, the mandatory Transforming Episode Accountability Model (TEAM) will be implemented on Jan. 1, 2026. TEAM is a five-year demonstration model that will determine whether bundling all costs of care for an episode into an episodic-based payment for several common, high-cost procedures will reduce costs while maintaining quality.
Proposed Changes to Outpatient and Ambulatory Sites of Care
The proposed 2025 outpatient prospective payment system (OPPS), ambulatory surgery center and physician fee schedule (PFS) payment rule sets are being finalized and will begin Jan. 1, 2025. The scope is broad, covering healthcare in all outpatient settings to allow for shifts between various sites of care. Several other pieces of legislation being debated will significantly impact pharmacy practices, from revenue streams, operations and clinical services standpoints and reflect a deeper dive into prior authorizations, pharmacy benefit managers, transparency and site-of-care changes, as well as the 340B program.
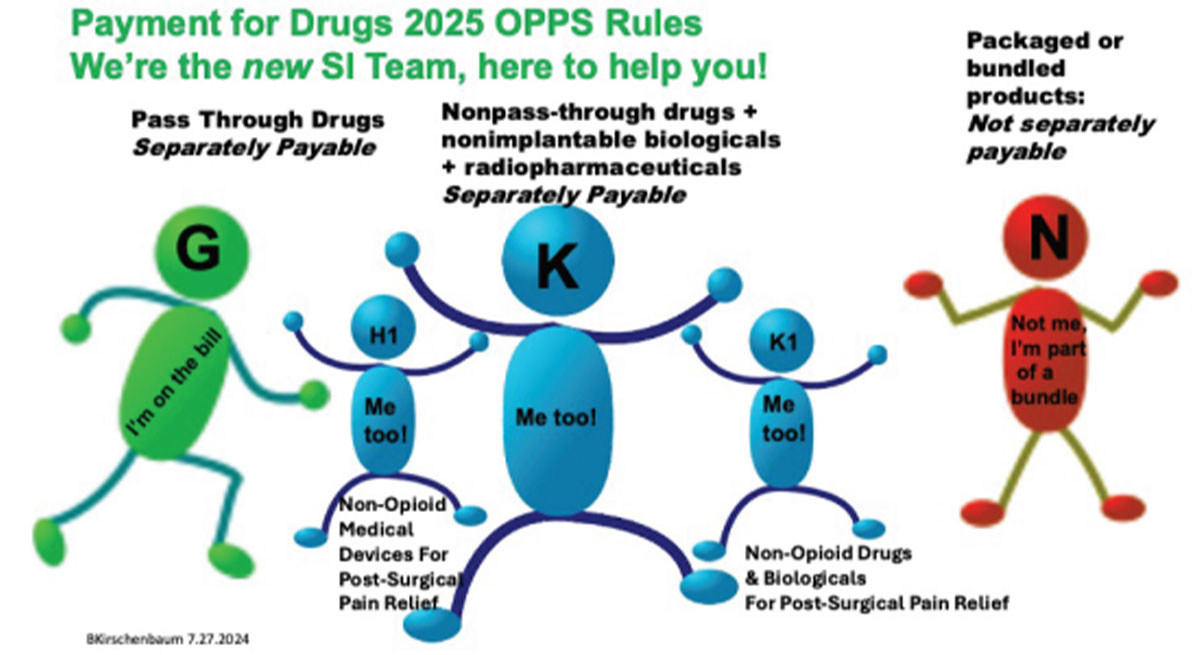
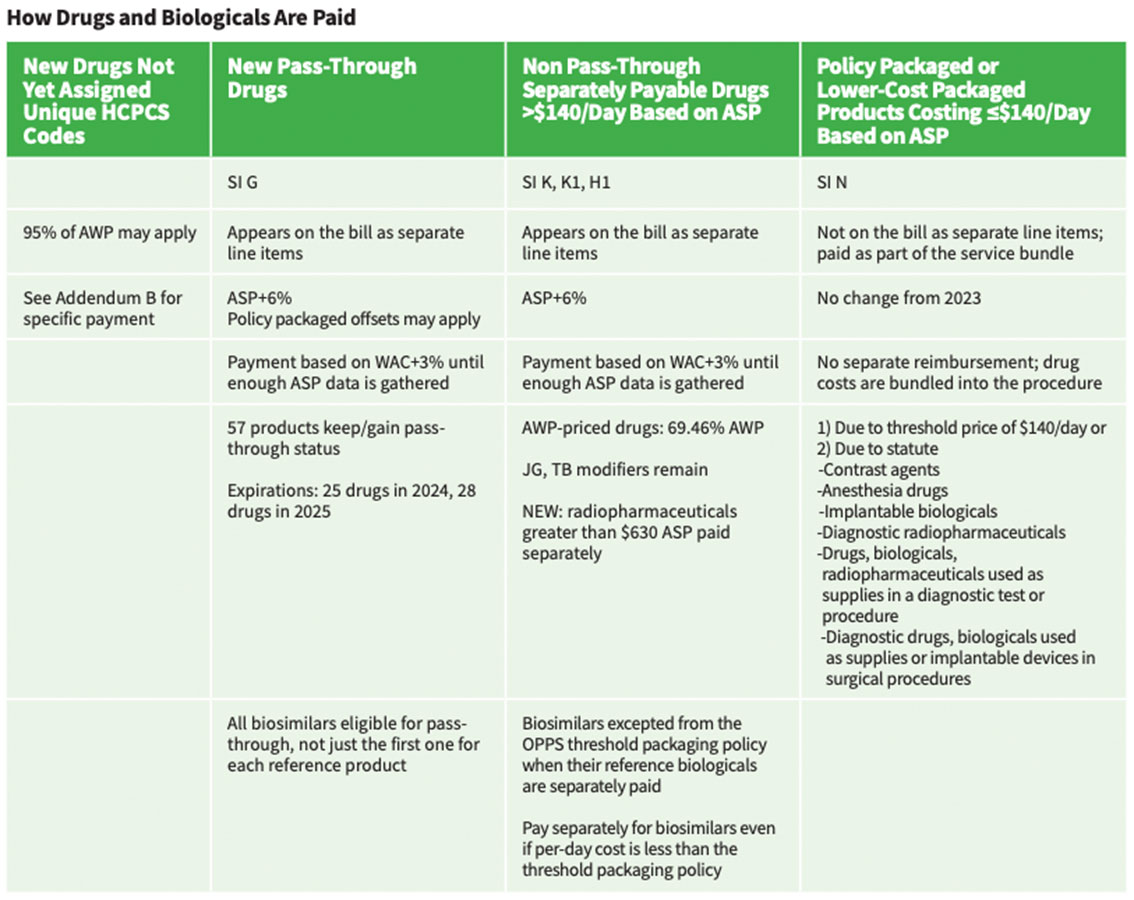
Under OPPS, the 2025 drug packaging threshold is proposed to be $140 average sales price (ASP) for all drugs, biologicals and therapeutic radiopharmaceuticals with a proposed separate payment for diagnostic radiopharmaceuticals when their per-day cost exceeds the proposed threshold of $630. OPPS rates have a variable mark-up determined each year, and are remaining at ASP+6%, with the exception of those covered by the Inflation Reducation Act (IRA) at ASP+8%. PFS rates are covered by statute and remain at ASP+6%. Drugs and biologicals are paid for in one of four ways (Table).
Pass-Through Payment Provisions
These require additional payment under Medicare Part B for current orphan drugs; current drugs and biologicals and brachytherapy sources used in cancer therapy; and current radiopharmaceutical drugs and biologicals. For at least two and up to three years, they also are provided for certain “new” drugs and biologicals whose cost is “not insignificant” in relation to OPPS payments for the procedures or services associated with the new drug or biological. For pass-through payment purposes, radiopharmaceuticals are included as “drugs.”
Provisions of the Inflation Reduction Act of 2022
Some provisions of the IRA of 2022, including the Medicare Drug Price Negotiation Program, Medicare Prescription Drug Inflation Rebate Program and the Medicare Part D Redesign Program, are underway and melded into the 2025 rule sets. This can be confusing, especially with regard to biosimilars. Here’s an example of how they apply:
• IRA establishes a payment rate for biosimilars under Medicare Part B during the initial period: For biosimilars furnished on or after July 1, 2024, the initial period payment rate is the lesser of the biosimilar’s wholesale acquisition cost (WAC) plus 3 percent or 106 percent of the reference product’s ASP. Subsequently this increased the Medicare Part B addon payment for qualifying biosimilars from 6 percent to 8 percent of the reference product’s ASP for a fiveyear period. During this period, the payment for such biosimilars would be the biosimilar’s ASP+8% of the ASP of the reference biological.
• Under the OPPS threshold packaging policy, biosimilars are excepted when their reference biologicals are separately paid. (The goal is to promote use as a lowercost alternative.) If the reference product cost per day falls below threshold, all biosimilars related to it would be similarly packaged regardless of whether their costs per day are above it.
Other Key Revisions and Requirements to Watch
• New status indicators (H1 for nonopioid medical devices for postsurgical pain relief and K1 for non-opioid drugs and biologicals for postsurgical pain relief) to identify products qualifying for separate payment under the new payment policy for non-opioid postsurgical pain management drugs, biologicals and devices to provide additional payments for access to non-opioid pain relief treatments until 2027
• Expanded coverage of hepatitis B vaccinations to beneficiaries who have not been previously vaccinated or whose vaccination status is unknown
• A new payment methodology for supplying and administering dependent care assistance programs such as preexposure prophylaxis (PrEP) for HIV consistent with ASP methodology (i.e., ASP+6%) (Use this PrEP payment methodology as it finalizes its national coverage determination moving PrEP coverage from Part D to Part B.)
• Revised eligibility requirements in the special enrollment period (SEP) for formerly incarcerated individuals (released on or after Jan. 1, 2025) to tie the eligibility for this SEP to the Social Security Administration’s determination that they are no longer incarcerated
• Codified requirement in the Consolidated Appropriations Act, 2023, to provide 12 months of continuous eligibility to children under the age of 19 in Medicaid and CHIP, with limited exceptions
• Updates to the conditions of participation (CoPs) for hospitals and critical access hospitals in an effort to advance the health and safety of pregnant, birthing and postpartum patients
• Separate payments to Indian Health Service (IHS) and tribal hospitals for high cost drugs furnished in hospital-based outpatient departments through an add-on payment, in addition to the all-inclusive rate (AIR) under the authorities used to calculate the AIR starting Jan. 1, 2025
• Exceptions to the Medicaid clinic services benefit four-walls requirement for IHS and tribal clinics, and, at state option, for behavioral health clinics and rural clinics
• Electronic prescribing for controlled substances
• Additional proposed provisions that will impact clinical and distributive services, some of which may be in an incident to arrangement depending on a state’s scope-of-practice limitations; payment for remote services that align OPPS payment for services furnished remotely to patients in their homes with payment under PFS includes payment for diabetes selfmanagement training, remote nutrition therapy and mental health services
• New flexibilities for opioid treatment programs (OTPs), including permanently allowing audio-only periodic assessments and allowing the OTP intake add-on code to be furnished via two-way audio video communications technology when billed for the initiation of treatment with methadone when clinically appropriate; payment increases apply to OTPs, as well as add-on codes for new U.S. Food and Drug Administration-approved opioid agonist and antagonist medications
References
1. CY 2025 Medicare Hospital Outpatient Prospective Payment System and Ambulatory Surgical Center Payment System Proposed Rule (CMS 1809-P). CMS news release, July 10, 2024. Accessed at www.cms.gov/newsroom/fact-sheets/cy-2025-medicare-hospital-outpatient-prospective-payment-system-andambulatory-surgical-center.