Agents That Block Plasma IgG Recycling: The Next Big Thing for IgG Antibody-Mediated Diseases?
- By Keith Berman, MPH, MBA
HERE IS A question that just might stump you: Is it possible a single monoclonal antibody-based therapeutic could effectively treat disorders as diverse as myasthenia gravis (MG), autoimmune mucocutaneous blistering diseases and immune thrombocytopenic purpura (ITP)? The answer is yes.
At least three investigational agents, all of which target neonatal Fc receptor (FcRn) on the surface of blood vessel endothelial cells, are currently in clinical testing. These FcRn inhibitors define a potential new therapeutic class that, if proven safe and effective, could represent an important new treatment option for IgG autoantibody and alloantibody-mediated diseases known to respond to administered IgG or to therapeutic plasma exchange.
FcRn Plays the Long-Acting Game
After human albumin, which accounts for more than two-thirds of total plasma protein, the four immunoglobulin classes (IgA, IgE, IgG and IgM) comprise the next most abundant plasma protein component. IgG alone accounts for 75 percent of circulating immunoglobulin and roughly 15 percent of total plasma protein. Combined, IgG and albumin account for a remarkable 80 percent to 90 percent of plasma protein.
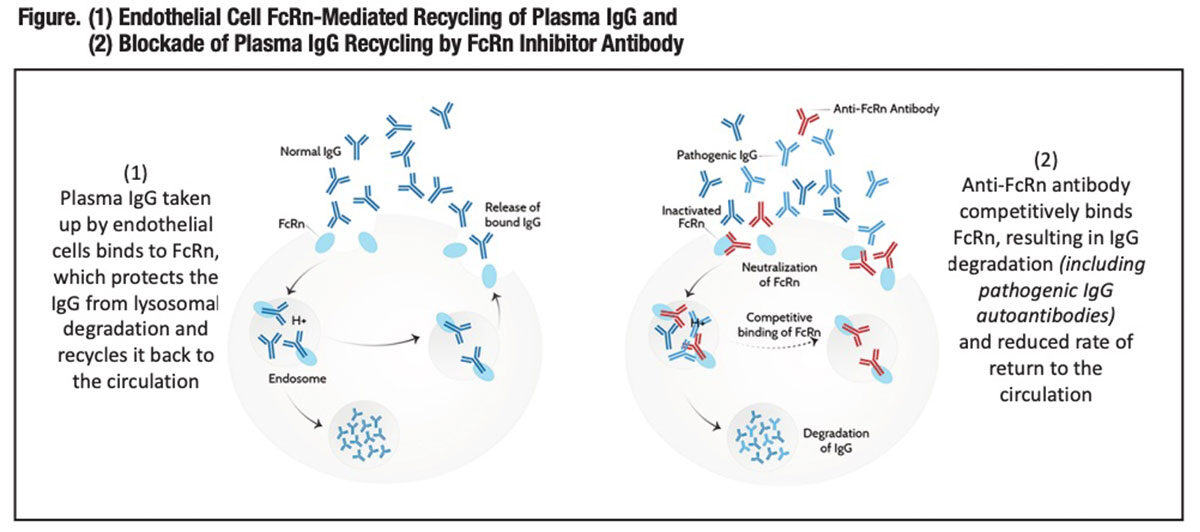
To maintain this high plasma IgG concentration, a “recycling” mechanism evolved that protects it from cellular catabolism, thus extending its total circulating half-life to 19 days to 23 days, compared to just five days to six days for IgA and IgM.1 That mechanism (Figure) is mediated by FcRn, a surface blood endothelial protein first identified for its role in the facilitated transport of IgG from the mother to the fetus or neonate (thus its name).
FcRn interacts with the constant tail region — the Fc region — of plasma IgG and internalizes it into recycling endosomes, protecting the IgG from degradation by cellular lysosomes. Still complexed with FcRn, IgG contained in endosomes migrates to the cellular surface, where by exocytosis it is released back into the circulation. In short, FcRn rescues IgG from cellular catabolism and recycles it into the circulation; thus the prolonged 19- to 23-day half-life.*
Drug development scientists eventually figured out this FcRn-mediated IgG recycling functionality can be “hijacked” to extend the half-life of therapeutic proteins with short intravascular persistence, in particular those that must be infused on an ongoing basis. Two such products, chronically self-administered by persons with hemophilia A and B to prevent bleeds, are ELOCTATE, a Bdomain-deleted factor VIII:Fc featuring a 50 percent longer half-life than factor VIII alone, and ALPROLIX, a factor IX:Fc with a threefold longer half-life than factor IX alone. Sometimes referred to as “biobetters,” these and other Fc fusion proteins significantly reduce the burden of self-injections and improve patient treatment compliance.
The Conceptual Basis for FcRn Inhibitors
The IgG-protective role of FcRn was first characterized more than 20 years ago by investigators who documented abnormally short IgG half-lives in genetically manipulated mice that don’t express FcRn.2,3 These FcRn “knockout mice” typically have fourfold to fivefold lower levels of circulating IgG as normal mice,4 and injected IgG is rapidly cleared. Study of a rare human genetic syndrome involving FcRn-disabling mutations further supports the role of FcRn as a homeostatic regulator of IgG levels: In two affected siblings from a consanguineous marriage, their abnormally low expression of FcRn correlated with a very low plasma level of IgG.5
It is also well-documented that FcRn is saturable by IgG. High administered doses of exogenous IgG in the form of intravenous immune globulin (IVIG) or subcutaneous immune globulin (SCIG) competitively saturate FcRn receptors, resulting in much-increased cellular catabolism of circulating endogenous IgG and a corresponding drop in endogenous IgG levels. FcRn saturation is a well-recognized mechanism of action of polyvalent human IG products: High-dose administered IgG equally drives down circulating levels of pathogenic IgG autoantibodies.6
Finally, there is a long-established treatment modality — therapeutic plasma exchange (TPE) — that effectively treats certain IgG-mediated disorders by physically reducing plasma IgG levels through 1:1 replacement of blood plasma with 5 percent human albumin. A single TPE procedure, with 1.5 plasma volumes replaced with 5 percent human albumin, acutely lowers plasma IgG by about 75 percent, followed by IgG synthesis-driven rebound.7 Patients commonly undergo five to six TPE procedures to drive down circulating levels of pathogenic IgG, with intermittent repeated TPE procedures to maintain the treatment effect. Both IG therapy and TPE have been shown to be similarly effective for treatment of three IgG autoantibody-mediated disorders: chronic inflammatory demyelinating polyneuropathy (CIDP), Guillain-Barré syndrome and MG.8,9
Motivated by this understanding, several research teams have designed monoclonal antibodies that target FcRn and block its IgG recycling functionality. The objective is fundamentally the same as TPE: to acutely or chronically reduce circulating levels of pathogenic IgG autoantibodies — whether characterized or not — that mediate disease.
FcRn Inhibitors in Development
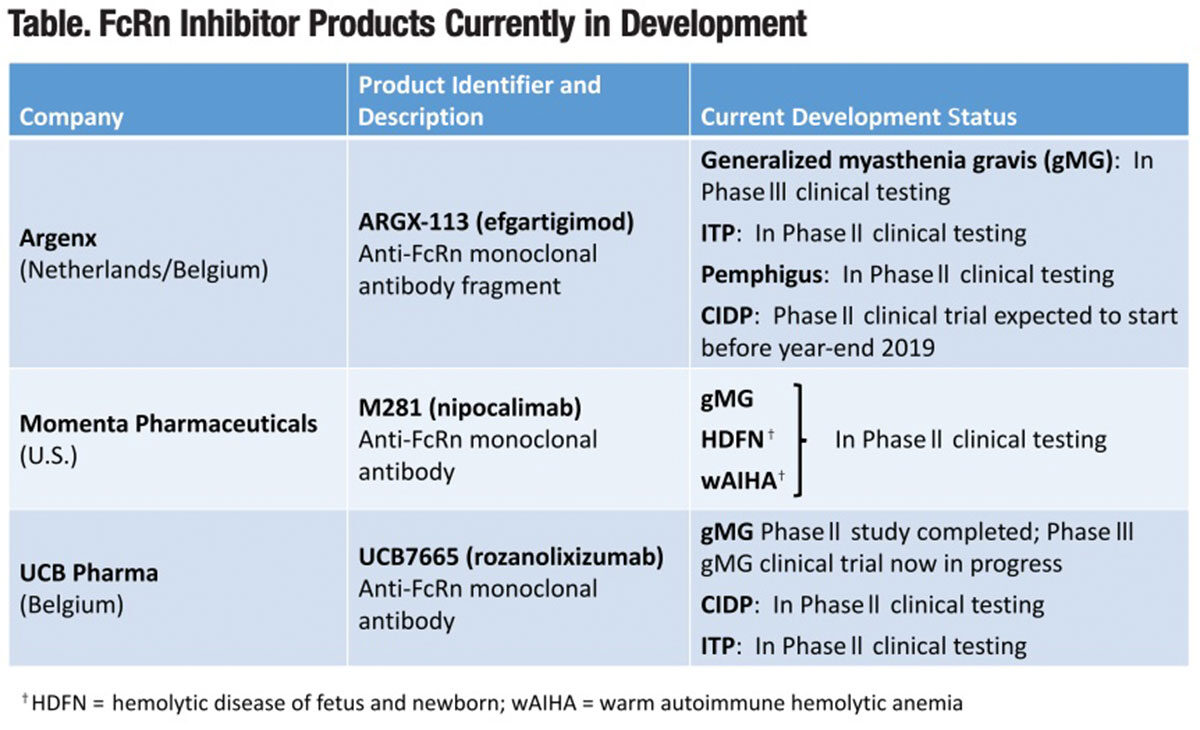
Three companies are currently evaluating or planning to evaluate their investigational anti-FcRn monoclonal antibody or antibody fragment products for the treatment of a half dozen immunity-based disorders (Table). All of these products selectively block FcRn-mediated IgG recycling, with the goal of reducing circulating levels of pathogenic IgG antibodies to achieve a prespecified therapeutic benefit.
Argenx. This Dutch/Belgian clinical stage biotechnology firm is developing efgartigimod (ARGX-113), a first-in-class antibody fragment that competitively binds FcRn in the same fashion as endogenous IgG. With repeated dosing, Argenx’s investigational FcRn inhibitor rapidly depletes circulating levels of IgG antibodies by as much as 85 percent. Argenx has completed a Phase I safety trial in healthy volunteers and is currently evaluating ARGX-113 for the treatment of four autoimmune disorders:
• Generalized MG (gMG). In a report in Neurology published in June, Phase II study findings in 24 patients randomized to ARGX-113 or placebo showed this investigational FcRn inhibitor was safe and well-tolerated, with a rapid decrease in IgG and anti-AChR autoantibody levels. All four efficacy scales demonstrated 75 percent of patients showed “a rapid and long-lasting disease improvement.”10 To fully evaluate ARGX-113’s efficacy, safety and tolerability, Argenx’s new randomized, double-blind, placebo-controlled Phase III ADAPT trial is currently recruiting up to 150 gMG patients at multiple sites in the U.S. and Europe.
• ITP. A randomized, double-blind, placebo-controlled Phase II trial is currently recruiting approximately 36 European patients to receive one or two doses of ARGX-113, in addition to standard-of-care treatment (e.g., oral corticosteroids, thrombopoietin receptor agonist). Initial testing has documented platelet count improvements across doses and ITP patient classifications, correlating with reduction in IgG levels.
• Pemphigus. A Phase II open-label, noncontrolled clinical trial is now recruiting 12 participants to evaluate the safety, pharmacokinetics, pharmacodynamics and efficacy of ARGX-113 in patients with mild to moderate pemphigus vulgaris and pemphigus foliaceus.
A seminal 2005 study11 showed experimental FcRn-deficient mice were resistant to cutaneous blister-inducing IgG antibodies, and levels of these pathogenic antibodies were significantly reduced relative to wild-type mice injected with them. Further, administration of high-dose human IgG (HDIG) drastically reduced circulating pathogenic IgG levels and prevented blistering, while in FcRn-deficient mice, no additional protective effect with HDIG was seen.**
Argenx is planning to launch a Phase II proof-of-concept trial to evaluate ARGX113 for the treatment of CIDP before the end of this year.
Momenta Pharmaceuticals. Cambridge, Mass.-based Momenta has developed nipocalimab (M281), a fully human anti-FcRn IgG1 monoclonal antibody. A randomized, double-blind, placebo-controlled Phase I trial in 50 healthy volunteers established multiple weekly doses achieved mean IgG reductions of about 85 percent from baseline, which were maintained at greater than or equal to 75 percent from baseline for up to 24 days. M281 was well-tolerated with no serious or severe adverse events and a low incidence of infection-related adverse events comparable to placebo treatment.12
The company is currently in progress with Phase II trials evaluating M281 for the treatment of three disorders:
•gMG. This PhaseII randomized, double-blind, placebo-controlled study, dubbed the “Vivacity-MG” study, is evaluating M281 in 60 U.S., Canadian and European patients with gMG, who have had an inadequate response to standard-of-care treatment.
• Hemolytic disease of the fetus and newborn (HDFN). In the instance of HDFN, the causal factor is not autoantibodies but one of three maternal alloantibodies (anti-RhD, anti-Rhc and anti-Kell) transferred to the fetus in utero. These alloantibodies attack fetal red blood cells, often resulting in serious morbidity or mortality. There are currently limited treatment options for HDFN, which affects an estimated 4,000 to 8,000 pregnancies each year in the U.S.
Momenta’s Phase II multinational open-label “Unity” study will enroll 15 pregnant women at high risk for early onset severe HDFN to receive intravenous infusions of M281. Its effectiveness will be assessed by examining the percentage of participants with a live birth at or after a fetal gestational age of 32 weeks, without the need for an intrauterine transfusion through their entire pregnancy. With the goal of expediting its development for this clinical application, the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation for M281 for treatment of HDFN.
• Warm autoimmune hemolytic anemia (wAIHA). Again with the support of FDA Fast Track Designation, Momenta has launched an adaptive Phase II/III clinical study to assess Momenta’s M281 product for the treatment of this rare autoantibody-mediated hemolytic disease. There are no approved treatments for wAIHA.
The adaptive design allows for modification of the study protocol based on interim analyses of the data. If successful, this Phase II/III study could serve as a pivotal study, making M281 the first labeled treatment option for wAIHA. Momenta expects to report top-line data from its gMG study in the second or third quarter of 2020, and from its HDFN and wAIHA studies in 2021.
UCB Pharma. Focused on treatments for immune and neurological disorders, this large multinational biopharmaceutical firm is currently investigating its high-affinity subcutaneous anti-FcRn monoclonal antibody, rozanolixizumab (UCB7665),13 in two autoimmune neurological disorders:
• gMG. In late 2018, UCB completed and reported top-line findings from a Phase II randomized, placebo-controlled proof-of-concept study in 43 North American and European patients. The study compared three infusions of placebo or UCB7665 administered over a four-week period, followed by a second dosing phase with continued observation until day 99. Infusions were safe and well-tolerated with clinically meaningful improvements seen across several prespecified disease-related endpoints over the entire duration of the study.
A 240-subject placebo-controlled Phase III study is now in progress to assess the safety and efficacy of two different dosage of UCB7665 in two dosage regimens in adult patients with gMG. This study is expected to be completed in early 2021.
• CIDP. A Phase II randomized, placebo-controlled study is currently enrolling 34 U.S. and Belgian CIDP patients to evaluate the efficacy, safety and tolerability of UCB7665.
• ITP. Earlier this year, UCB reported Phase II study findings in 54 primary ITP patients treated with UCB7665.14 No serious treatment-related adverse events or treatment discontinuations due to adverse events were reported. The platelet response increased with increasing dose intensity: 33 percent at the 4 mg/kg and 7 mg/kg doses, 50 percent at the 10 mg/kg dose and 67 percent at the 15 mg/kg dose. Based on these findings, study investigators are now dosing ITP patients at once weekly doses of 20 mg/kg.
Gazing Into the Future
Whether these FcRn inhibitors are safe and effective for the treatment of gMG, CIDP, ITP, pemphigus, wAIHA, HDFN and potentially other IgG autoantibody and alloantibody-mediated autoimmune disorders obviously awaits results of ongoing and future patient trials. But if shown to be safe and effective, these agents could be a viable therapeutic option for certain patients currently treated with IG and plasma exchange.
Individual patient considerations and published clinical outcomes will always dictate the treatment prescribed for a particular disease. But if shown to be safe and effective, FcRn inhibitors could be a viable treatment option in particular for patients with IG tolerability issues, as well as for those who are poor TPE candidates due to difficult venous access or some other contraindication.
Will FcRn inhibitors prove to be effective for just a few isolated disorders, or for a diverse spectrum of IgG antibody-mediated diseases? Or, will they end up falling short of expectations? As pivotal study results are reported over the next two to three years, we can look forward to the much-anticipated answers.
* FcRn additionally binds and facilitates recycling of circulating human albumin, which has a similar 19- to 21-day half-life.
** The investigators further concluded these findings both 1) support an FcRn saturation/pathogenic IgG degradation mechanism for high-dose IVIG in autoimmune skin blistering diseases, and 2) rule out the possibility that IVIG contains anti-idiotypic antibodies that neutralize blistering disease-inducingIgG antibodies.
References
- Lobo ED, Hansen RJ and Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci 2004 Nov;93(11):2645-68.
- Ghetie V, Hubbard JG, Kim JK, et al. Abnormally short serum halflives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol 1996 Mar;26(3)690-6.
- Israel EJ, Wilsker DF, Hayes KC, et al. Increased clearance of IgG in mice that lack beta 2-microglobulin: possible protective role of FcRn. Immunology 1996 Dec;89(4):573-8.
- Chaudhury C, Mehnaz S, Robinson JM, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med 2003;197(3):315-22.
- Waldmann T, Terry W. Familial hypercatabolic hypoproteinemia. A disorder of endogenouscatabolism of albumin and immunoglobulin. J Clin Invest 1990;86(6):2093-8.
- Yu Z, LennonVA. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. N Engl J Med 1999 Jan 21;340(3):227-8.
- Brecher ME. Plasma exchange: Why we do what we do. J Clin Apher 2002;17:207-11.
- Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice — Evidence-based approach from the writing committee of the American Society for Apheresis: The eighth special issue. J Clin Apher 2019 Jun;34(3):171-354.
- Barth E, Nouri MN, Ng E, et al. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology 2011 Jun 7;76(23):2017-23.
- Howard JF, Bril V, Burns TM, et al. Randomized phase 2 study of RcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology 2019 Jun 4;92(23):e2661-e2673.
- LiN, Zhao M, Hilario-Vargas J,etal. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest 2005;115(12):3440-50.
- Ling LE, Hillson JL, Tiessen RG,et al. M281, an anti-FcRn antibody: Pharmacodynamics, pharmacokinetics, and safety across the full range of IgG reduction in a first-in-human study. Clin Pharmacol Ther 2019 Apr;105(4):1031-9.
- Smith B, Kiessling A, Lledo-Garcia R, et al. Generation and characterization of a high affinity anti-human FcRn antibody, roxanolixizumab, and the effects of different molecular format on the reduction of plasma IgG concentration. MAbs 2018 Ot;10(7):1111-30.
- Robak T, Kaźmierczak M, Jarque I, et al. Safety and efficacy of an anti-FcRn antibody, rozanolixizumab, in patients with primary immune thrombocytopenia: Interim results of a Phase II, multiple-dose study. Abstract#S850. Presented at the 24th European Hematology Association Annual Congress, June 15, 2019.; Amsterdam, The Netherlands.