Grifols’ AMBAR Study Findings: Albumin Plasma Exchange May Reduce Progression of Moderate Alzheimer’s Disease
- By Keith Berman, MPH, MBA
IN WHAT COULD prove to be the first-ever demonstration of an effective disease-modifying treatment for Alzheimer’s disease (AD), top-line findings from Grifols’ Phase IIb Alzheimer Management by Albumin Replacement (AMBAR) clinical trial found long-term plasmapheresis with albumin replacement — plasma exchange — importantly reduced disease progression in a prespecified subset of patients with AD of moderate severity.
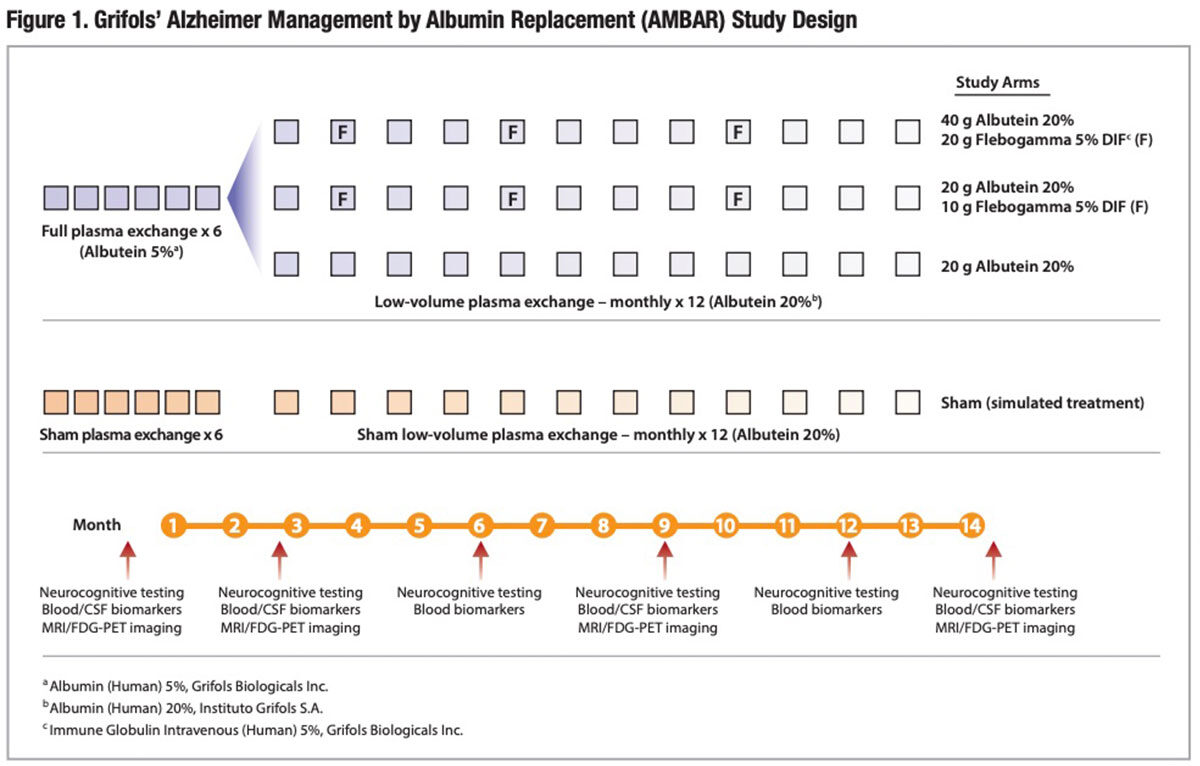
Forty-one participating treatment sites — 20 in Spain and 21 in the United States — recruited 496 patients age 55 years to 85 years with mild or moderate AD. Of these, 347 patients were randomized to a placebo arm receiving sham treatments, or to one of three arms treated with a series of six weekly conventional plasma exchange procedures with 5% albumin replacement, followed by 12 monthly low-volume plasma exchange procedures with 20% albumin replacement (Figure 1). Two of the three plasma exchange/plasmapheresis treatment arms additionally received three infusions of 10 or 20 grams of intravenous immune globulin (IVIG) over the 14-month study period, while the third arm did not receive replacement IVIG.
The primary study outcomes were changes from baseline to end-of-study treatment month 14 in well-validated scales of cognition (ADAS-Cog) and activities of daily living (ADCS-ADL). The study employed both a randomized and double-blind design to ensure neither patients nor evaluators knew whether subjects were receiving active treatment or placebo treatment.
Plasma Exchange Reduces Progression in Moderate Disease
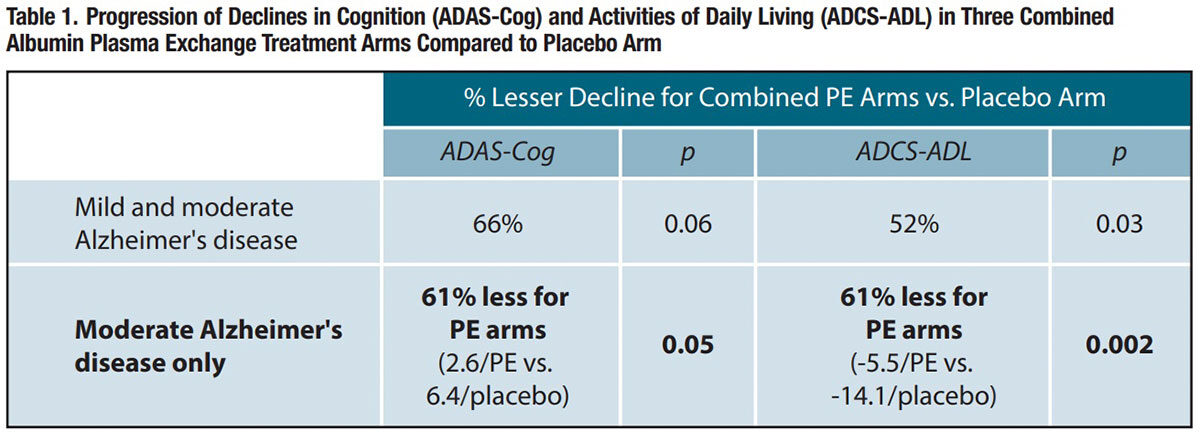
In an evaluation of all subjects regardless of disease severity, the combined arm including all patients treated with albumin plasma exchange experienced 66 percent less decline in the ADAS-Cog scale over the period from baseline to 14 months than the placebo arm, nearly reaching statistical significance (p = 0.06); for the ADCS-ADL scale, the combined albumin plasma exchange arm had a statistically significant 52 percent less decline than the placebo arm (p = 0.03).
The investigators then conducted the same analyses after stratifying on baseline disease severity. In prespecified patients with mild AD at baseline, a consistent delay in progression of disease was observed in the treatment arms, and a similar pattern was observed in the placebo arm. Differences in cognitive and functional decline tended to favor the combined active treatment arms relative to the placebo arm, but did not reach statistical significance. Therefore, the investigators suggested that “more follow-up time is needed to observe disease progression in milder disease.”
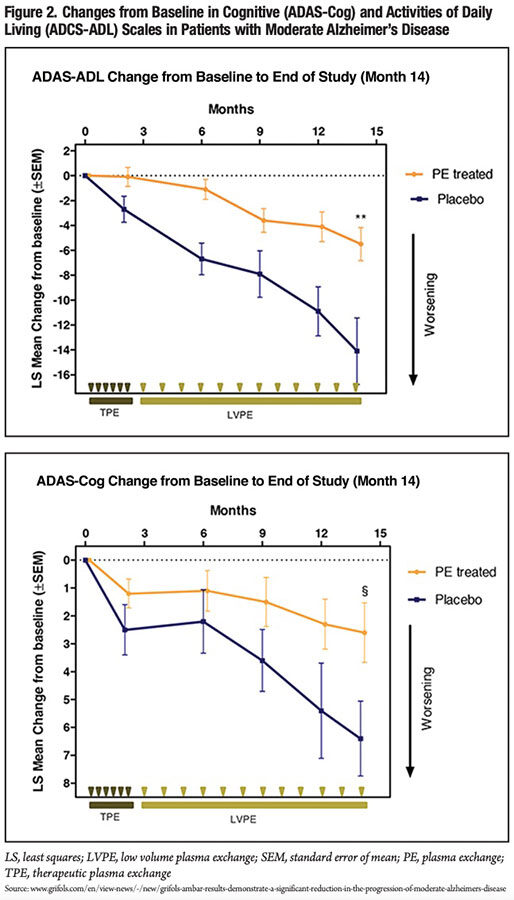
Conversely, over the 14-month study period, patients with moderate AD who were treated with albumin plasma exchange experienced 61 percent less decline in both the ADAS-Cog and ADCS-ADL scales than patients randomized to placebo (Table 1 and Figure 2). The difference between combined albumin plasma exchange arms and the placebo arm was statistically significant for the ADAS-Cog scale, and highly significant for the ADCS-ADL scale. When analyzed by plasma exchange combination type, all three treatment combinations for moderate AD patients reached statistical significance for ADCS-ADL relative to the placebo arm.
“The treatment effect observed in the group with moderate severity is remarkable and those findings open new avenues…that have the potential to offer Alzheimer’s disease patients a new modality of treatment,” said Oscar Lopez, MD, director of the Alzheimer’s Disease Research Center at the University of Pittsburgh.
Detailed safety and efficacy data are anticipated shortly in a full published report, but Spanish co-investigator Mercé Boada, MD, PhD, characterized the albumin plasma exchange procedures as “safe and feasible,” citing more than 1,000 plasma exchange procedures performed at her center in Barcelona and close to 5,000 in the entire study.
The Therapeutic Principle Behind Plasma Exchange
Given the long and diverse list of investigational drugs intended to slow AD progression that failed in large-scale clinical trials, no one can be faulted for not getting too excited over these promising findings. Yet the principle that underpins a chronic regimen of plasmapheresis with albumin replacement to battle AD is compelling:
- Extensive evidence points to an etiologic role of certain neurotoxic amyloid-β (Aβ) peptides, which are able to cross between the circulation and brain tissue through the blood-brain barrier (BBB). Aβ aggregates that accumulate as neuritic plaques are a histopathological hallmark of AD
- As much as 90 percent of circulating Aβ in healthy individuals is not free in plasma but instead is bound to albumin, a 67-kilodalton nonglycosylated protein that accounts for more than one-half of total protein in plasma and cerebrospinal fluid (CSF).
- Albumin undergoes glycation with normal aging, attenuating its physiologic Aβ binding capacity; eventually, this results in increased levels of unbound neurotoxic Aβ in the plasma and CSF.
- Glycation attenuates antioxidant and anti-inflammatory properties mediated by human albumin; a chronic pro-inflammatory state is correlated with a number of common degenerative diseases of the elderly, including AD.
- Both brain and plasma levels of glycated albumin have been found to be significantly higher in persons with AD than in age-matched controls.
Plasmapheresis physically removes a portion of the patient’s “old” less-functional glycated albumin, which is replaced with purified human albumin sourced from plasma collected from relatively young, healthy plasma donors. This infused replacement albumin both retains its Aβ binding capacity and appears to have no quantifiable levels of bound Aβ.
This plasma exchange procedure is repeated on a regular chronic basis, with the goal of reducing brain Aβ burden by exploiting the dynamic equilibrium between the brain and blood plasma. Each time the patient’s old glycated, Aβsaturated albumin is removed by apheresis and replaced with fresh, Aβ-binding albumin, a brain-plasma concentration gradient is created by the resulting drop in plasma Aβ concentration. Uncomplexed Aβ residing in the brain and CSF is mobilized by this new concentration gradient and transits across the BBB and into the circulating plasma until a new brain-plasma Aβ equilibrium is established. All of this is established science documented in numerous studies by Grifols collaborators and others.
Why Plasma Exchange?
Investments now amounting to billions of dollars in development of failed experimental AD treatments have not deterred the drug industry from continuing the quest. There is an urgent unmet need for effective treatments that can stabilize or meaningfully delay progression of this disease. Some 5.7 million Americans now live with AD, a number projected to increase to nearly 14 million people by 2050. Atop the enormous human toll is an estimated $277 billion national cost associated with this disease, which could rise as much as four-fold by 2050. Some 70 proposed disease-modifying agents are currently in clinical development for AD, including a number of sophisticated new anti-amyloid monoclonal antibodies and anti-aggregation agents.
Grifols’ proposed mechanism of action to treat AD presents a stark contrast with all the highly targeted anti-amyloid drugs of the past and present: physical removal of neurotoxic amyloid proteins with chronic plasmapheresis and albumin replacement therapy. It is elegant in its simplicity. And therapeutic plasma exchange has a decades-long clinical track record as a safe and highly effective treatment for dozens of disorders, including many for which the circulating etiologic agent removed by this procedure is unknown.
Next Steps
While the top-line AMBAR study findings are highly encouraging, there is a long list of would-be AD treatments for which strong preliminary efficacy signals observed in Phase II trials could not be replicated in larger pivotal studies. “More research is needed in a larger study so we know if this is an effective procedure for the treatment of Alzheimer’s dementia,” said Alzheimer’s Association Scientific Director Maria Carrillo, PhD.
At the conclusion of his presentation of AMBAR study findings at an international research conference last October, Grifols Medical Director Antonio Páez told attendees that the company plans to sponsor a new trial to definitively answer whether the AD progression can be slowed or stabilized with plasma exchange. Stay tuned.
References
- Roche M, Rondeau P, Singh, NR, et al. The antioxidant properties of serum albumin. FEBS Letters 2008;582(13):1783-7.
- Howcroft TK, Campisi J, Louis GB, et al. The role of inflammation in age-related disease. Aging(Albany NY) 2013 Jan;5(1):84-93.
- Ramos-Fernández E, Tajes M, Palomer E, et al. Posttranslational nitro-glycative modifications of albumin in Alzheimer’s disease: implications in cytotoxicity and amyloid-β peptide aggregation. J Alzheimers Dis 2014;40(3):643-57.
- Alzheimer’s Association. Accessed 10/29/2018 at www.alz.org/alzheimers-dementia/facts-figures.
- Cummings J, Lee G, Ritter A, et al. Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement (NY) 2018 May 3;4:195-2014.
- Schwartz J, Padmanabhan A, Aqui N, et al. Guidelines on the use of therapeutic apheresisin clinical practice—Evidence-based approach from the writing committee of the American Society for Apheresis: The seventh special issue. J Clin Apher 2016;31(3):149-338.
