Innovations in Bioidentical Hormone Replacement Therapy
Touted as a more natural approach to hormone replacement therapy, this plant-based anti-aging remedy has evolved from an unregulated safety concern to a mainstay of modern medicine.
- By Trudie Mitschang
HORMONES PLAY a huge role in how the body functions on every level, from weight management and mental alertness to stamina, bone health and quality of sleep. With aging, optimal hormone levels begin to decline or even deplete, so it’s no surprise that hormone replacement therapy (HRT) has emerged as an increasingly popular method to turn back the clock and improve quality of life as people age.
HRT is typically used to treat symptoms associated with menopause, including hot flashes, accelerated skin aging, vaginal dryness, decreased muscle mass and osteoporosis. These symptoms are mostly caused by low levels of estrogen and progestogens.
While the popularity of HRT has surged in recent years, it is far from a new idea. History documents early attempts at hormone therapy back to ancient China, where aging Chinese women routinely ingested young women’s dried urine to counteract health symptoms associated with menopause (the belief was that young women’s urine contained high levels of metabolic waste products such as progesterone, estrogen and testosterone).1
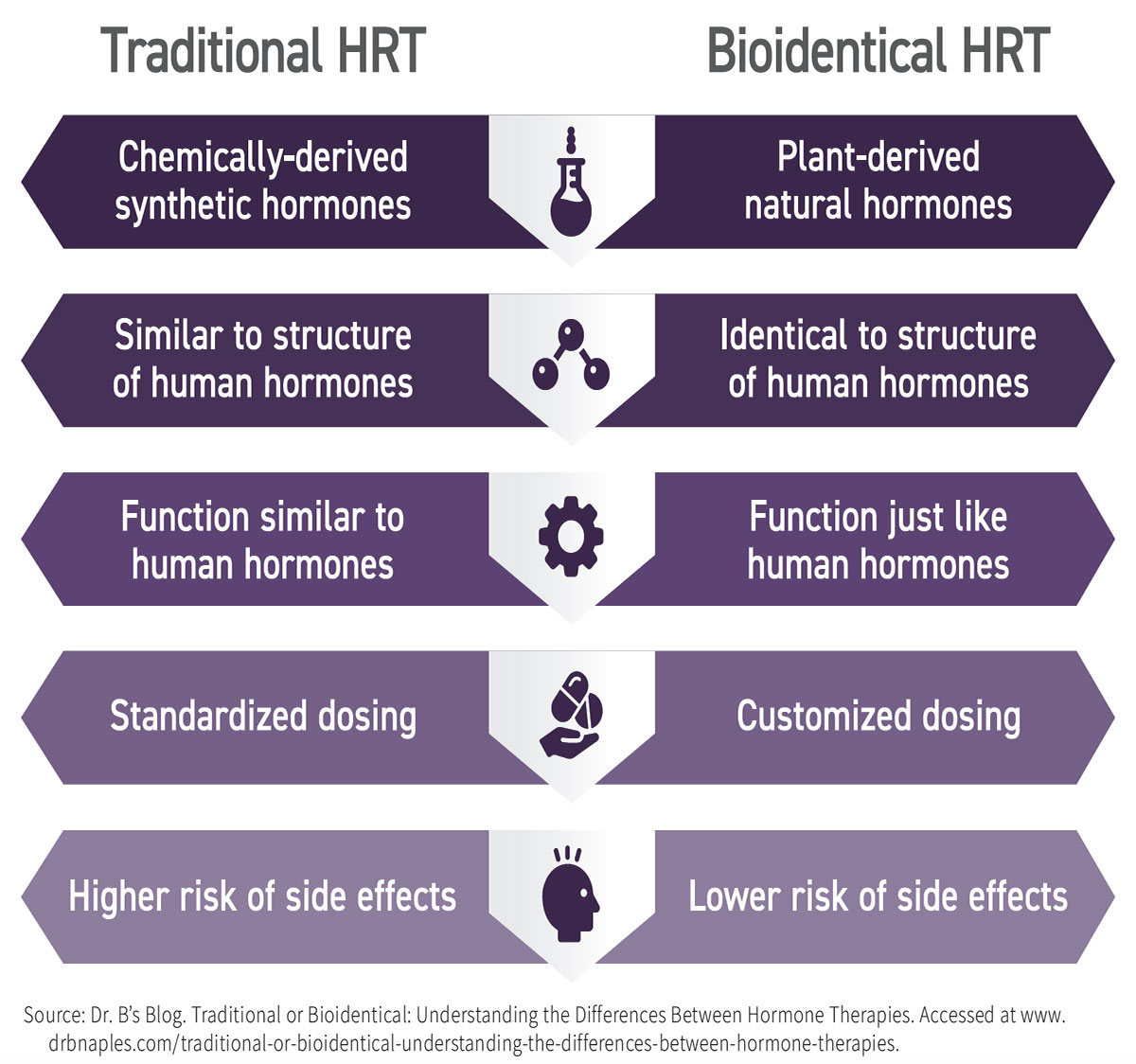
Fast forward to modern times and HRT can be traced to the mid-20th century when researchers began investigating it for menopausal women. Initially, synthetic hormones derived from the urine of pregnant horses were commonly prescribed, but concerns arose regarding their safety, including research findings linking HRT with an increased risk of breast cancer and heart disease. This led to the exploration of bioidentical HRT (BHRT). BHRT uses manufactured compounds said to have exactly the same chemical and molecular structure as hormones produced in the human body and are derived from plants.
In the 1980s and 1990s, interest in BHRT surged as more studies highlighted its potential benefits and safety profile. Patients who sought alternatives to traditional HRTs were drawn to the idea of what was promoted as a “natural” alternative. By the early 2000s, BHRT had gained widespread attention, with clinics across the country offering these therapies to address various hormonal imbalances, including menopause, thyroid disorders and adrenal insufficiency. And with growing demand, medical professionals continued to refine BHRT protocols and formulations to optimize patient outcomes.2
Safety and Risk Factors
BHRT uses chemicals derived from plants such as soy and wild yams that contain compounds called phytoestrogens, which are chemically similar to estradiol, a form of estrogen produced in the ovaries. In a laboratory, scientists can extract these phytoestrogens and chemically convert them into bioidentical hormones that have the same structure and function as the hormones produced by the body. The primary hormones used in BHRT are estrogen, progesterone and testosterone, which can be administered in various forms, including pills, patches, creams, gels and injections. BHRT is often used to treat hormonal imbalances and relieve symptoms of menopause in women or andropause in men.
This similar makeup of bioidentical hormones to those found in the human body is believed to enhance their effectiveness and reduce the side effects associated with synthetic hormones. Erika Schwartz, MD, a prominent advocate of BHRT, states, “Bioidentical hormones are more effective because they mimic the hormones our bodies naturally produce.”
When it comes to safety, doubts were raised over the safety of conventional HRT in 2002, when studies linked their extended use to a risk of breast cancer and heart disease. But is BHRT really a safer alternative? Detractors contend there have not been sufficient studies to evaluate the safety of soy- and yam-based hormones. Other concerns include the risk that hormones compounded by a specialty pharmacy can be inconsistent in terms of strength and dosage. Here is what the research shows:
• A 2009 California study asserted that the use of natural hormones is associated with a lower risk of breast cancer and cardiovascular disease, and that they are more effective than synthetic and animal-derived versions. (It also conceded that scientific trials were needed to investigate these differences.)3
• A 2012 clinical trial in Denmark demonstrated that healthy women taking BHRT for a decade immediately after menopause had a reduced risk of dying from heart disease.3
• The conclusion of a recent study published in the medical journal JAMA states: “The benefits of hormone therapy for the treatment of menopause symptoms outweigh the risks.”4 “Among women below the age of 60, we found hormone therapy has low risk of adverse events and [is] safe for treating bothersome hot flashes, night sweats and other menopausal symptoms,” said study author JoAnn Manson, MD, MPH, DrPH, chief of preventive medicine at Brigham and Women’s Hospital. “This is a departure from the advice many women have been given in the past.”4
The recent study’s analysis is based on two decades of follow-up data from the 1991 Women’s Health Initiative study, which followed thousands of women taking HRT. The study was halted after it found that women taking Prempro (which is a combination of estrogen and progestin) had higher risks of breast cancer and stroke. “The findings were surprising,” Dr. Manson notes, pointing out that the reason the randomized trial was conducted was because scientists were trying to determine if hormone therapy decreased the risk of heart disease and other conditions.
After the initial findings came out, many women abruptly stopped the therapy. Prescriptions plummeted, and many healthcare providers hesitated to recommend hormone therapy. But menopause experts say it’s time to reconsider, because there’s a lot known now that wasn’t known two decades ago. Most significantly, there are now different types of hormones — delivered at lower doses — that are shown to be safer.
“Women should know that hormone therapy is safe and beneficial,” says Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University Feinberg School of Medicine. In hindsight, Dr. Streicher believes the Women’s Health Initiative study was flawed and that some of the risks that were identified were linked to the type of hormones women were given, as well as the age of the women enrolled (they were all over 60). “We know that there is a window of opportunity when it is the safest to start hormone therapy and that you get the most benefit. That window is typically between ages 50 and 60,” she adds.4
Exploring the Options
Individuals interested in BHRT have a number of choices when it comes to choosing the treatment option that is right for their specific symptoms:
• Lab-made bioidentical hormones that are chemically similar to the hormones in the body but are derived from a plant steroid found in soy and wild yams.
• Compounded bioidentical hormones custom-created at a compounding pharmacy.
• Food and Drug Administration (FDA)-approved bioidentical hormone products manufactured by pharmaceutical companies and prescribed under brand names.
All bioidentical hormones begin in a laboratory where phytoestrogens are extracted from plants. Compounding pharmacies and pharmaceutical companies both obtain those same bioidentical hormone products from the same chemical laboratory companies, but their paths then diverge. The pharmaceutical companies prepare their products (pills, gels, creams or patches) under strict FDA regulations. The compounding pharmacies take the same bioidentical hormone preparations and develop them into their own preparations based on individual lab tests and prescriptions. The advantage of the compounding pharmacy is that it can tailor the dosage delivered to the patient, while pharmaceutical companies have a more restricted dose range of the hormones they can offer, making the prescriptions more of a one-size-fits-all approach. In terms of oversight, pharmaceutical companies are under strict safety rules, while compounding pharmacies must report only to their state pharmacy boards and do not require FDA approval.
A compounded hormone product may contain one hormone or a combination of a few different hormones. For example, some healthcare providers will combine more than one type of estrogen together, and depending on the woman’s symptoms, they may add progesterone as well. Compounded bioidentical hormone therapies (CBHTs) may be a good choice for those unable to take an FDA-approved product due to an allergy (such as peanut oil) or for those who did not find symptom relief from a commercial product. In some cases, a healthcare provider might prefer a compounded product and try that as a first course of treatment.
A study analyzing why women choose compounded BHRT found women are “not only seeking alternatives to conventional pharmaceuticals, but alternatives to conventional care where their menopausal experience is solicited, their treatment goals are heard, and they are engaged as agents in managing their own menopause.”5 The study concluded that “women making menopause treatment decisions of all kinds would benefit from greater shared decision-making in the clinical context in which they are explicitly invited to share their experiences, priorities and preferences. This would also provide an opportunity for clinicians to discuss the pros and cons of conventional HT, CBHT and other approaches to managing menopause.”
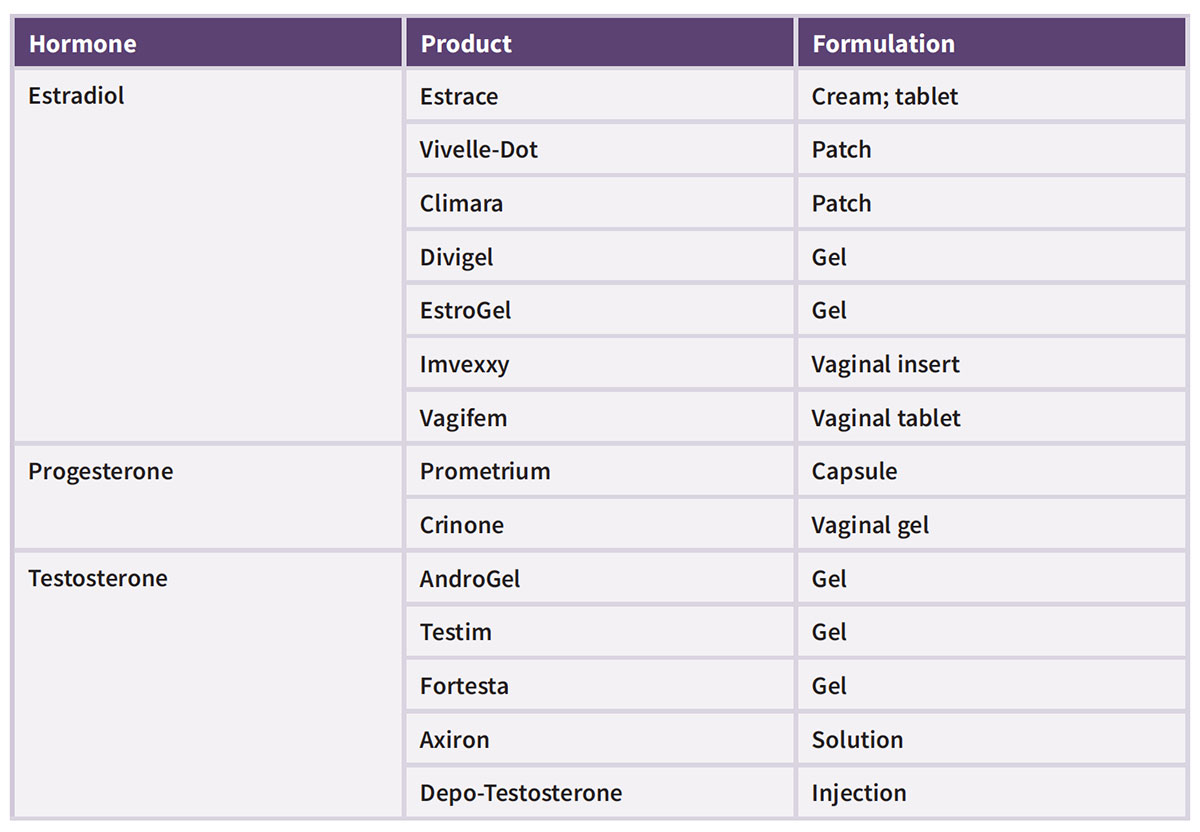
Those with safety and efficacy concerns may prefer FDA-approved bioidentical hormones that are manufactured under strict guidelines by pharmaceutical companies prior to being released into the market. In 2018, FDA granted its first BHRT approval, a combination of estradiol and progesterone for moderate to severe vasomotor symptoms associated with menopause. The approval of Bijuva came on the heels of a clinical trial that demonstrated a combination of 17beta-estradiol and progesterone appeared to be safe and effective for reducing hot flash frequency and severity in menopausal women with a uterus.6 Today, there are a number of FDA-approved hormone products that meet the definition of bioidentical. (See Table for list of available products.)
What’s on the Horizon?
HRT has been proven to help any number of age-related health issues, and in a culture in which anti-aging remedies are increasingly in high demand, its popularity will likely continue to grow. Moving beyond the current use cases, new and promising research is now linking HRT to a reduced risk of Alzheimer’s disease and dementia. “There’s a window of opportunity,” said lead study author Lisa Mosconi, PhD, director of the Alzheimer’s Prevention Program and the Women’s Brain Initiative at Weill Cornell Medicine in New York City. “Hormones work best for the brain when taken in midlife in presence of menopausal symptoms to support women through the menopause condition.”7
In fact, the brain has a higher chance of being protected if hormone replacement is started soon after menopausal symptoms begin, according to the analysis recently published in the journal Frontiers in Aging Neuroscience.8 The length of time a woman undergoes therapy also matters: As long as a woman began hormones while she was in menopause, there was a 26 percent reduced risk of dementia if hormones were taken for more than 10 years, the study found.
“While there is not a clear one-size-fits-all approach, in the right woman, at the right dose and for the right duration of time, I believe that hormone replacement therapy can be one of our most powerful tools to reduce a woman’s risk for cognitive decline and to slow down Alzheimer’s pathology,” said Richard Isaacson, MD, director of research at the Institute for Neurodegenerative Diseases in Florida. “I believe this may be especially true for women with one or more copies of the APOE4 genetic variant, which is present in about 25 percent of people. It’s essential for neurologists and primary care physicians to work closely with gynecologists … and monitor treatment outcomes over time.”7
This study and others show that BHRT continues to offer healthcare breakthroughs, and researchers continue to uncover potential treatment outcomes. Dr. Streicher agrees: “Hormone therapy is beneficial way beyond the benefits to just helping with hot flashes. Ongoing research points to protection against bone loss and even heart disease.”4
Patients considering BHRT should engage in thorough discussions with their healthcare providers, weigh the potential benefits and risks, and consider their personal health history and needs. Ongoing research and more rigorous clinical trials will be essential in providing clearer guidance and improving the safety and efficacy of what is becoming a common defense in the arsenal against aging.
References
1. Medical Health Institute. History of Bioidentical Hormones. Accessed at medicalhealthinstitute.com/history-of-bio-identical-hormones.
2. Johnson, S. A Brief History of BHRT. Restorative Health, March 18, 2024. Accessed at restorative-health.com/brief-history-of-hormone-replacement-therapy.
3. Hardy, F. Daily Mail Interview: Dr. Erika Schwartz. Accessed at www.dailymail.co.uk/femail/article-3249709/Can-woman-help-make-menopause-best-days-life-Meet-doctor-s-won-host-high-flying-women.html.
4. Manson, JE, Crandall, CJ, Rossouw, JE, et al. The Women’s Health Initiative Randomized Trials and Clinical Practice: A Review. JAMA, 2024;331(20):1748-1760. Accessed at jamanetwork.com/journals/jama/article-abstract/2818206?resultClick=24.
5. Thompson, JJ, Ritenbaugh, C, and Nichter, M. Why Women Choose Compounded Bioidentical Hormone Therapy: Lessons from a Qualitative Study of Menopausal Decision-Making. BMC Womens Health, 2017; 17: 97. Accessed at www.ncbi.nlm.nih.gov/pmc/articles/PMC5625649.
6. FDA Approves Bioidentical Hormone Therapy for Menopausal Hot Flashes. Healio, Oct. 29, 2018. Accessed at www.healio.com/news/endocrinology/20181029/fda-approves-bioidentical-hormone-therapy-for-menopausal-hot-flashes.
7. LaMotte, S. Sweet Spot for HRT May Reduce Dementia Risk by Nearly a Third, Study Says. CNN, Nov. 2, 2023. Accessed at www.cnn.com/2023/11/02/health/hormone-replacement-dementia-wellness/index.html.
8. Nerattini, M, Jett, S, Caroline, A, et al. Systematic Review and Meta-Analysis of the Effects of Menopause Hormone Therapy on Risk of Alzheimer’s Disease and Dementia. Aging Neuroscience, Oct. 22, 2023. Accessed at www.frontiersin.org/journals/aging-neuroscience/articles/10.3389/fnagi.2023.1260427/full#ref21.
