Industry News
Research, Science & Manufacturer Updates
Biosimilars Articles
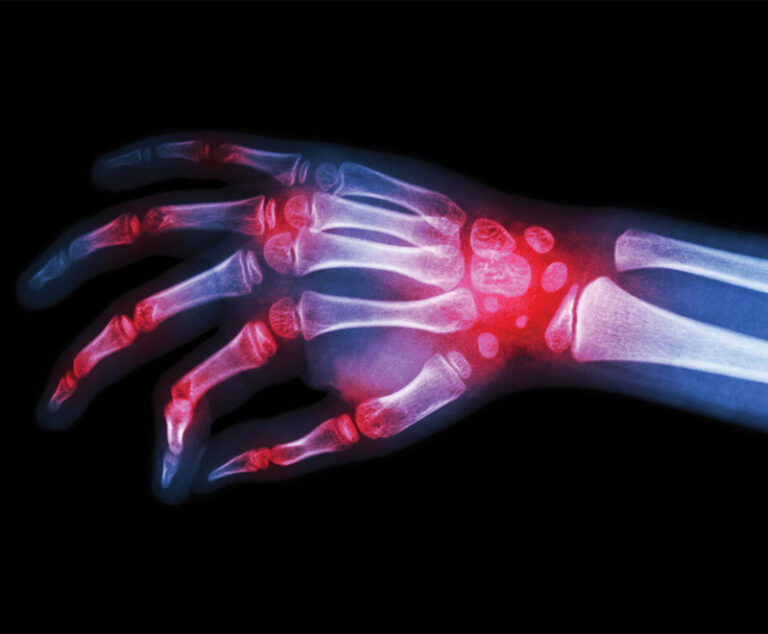
The U.S. Food and Drug Administration has approved Tyenne (tocilizumab-aazg), a biosimilar referencing tocilizumab (Actemra; Genentech), to treat multiple autoimmune diseases, including rheumatoid arthritis and juvenile idiopathic arthritis.
FDA has approved Alvotech’s Simlandi (adalimumab-ryvk) as the third interchangeable Humira biosimilar.
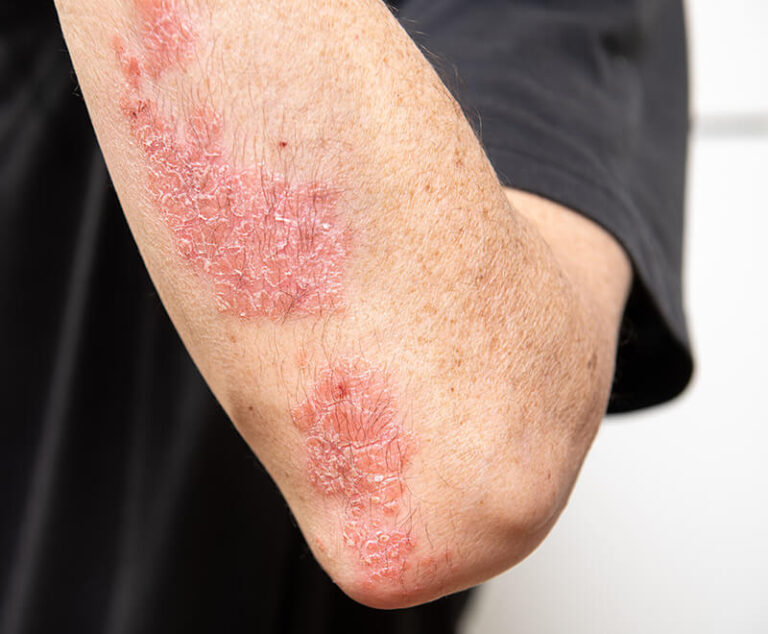
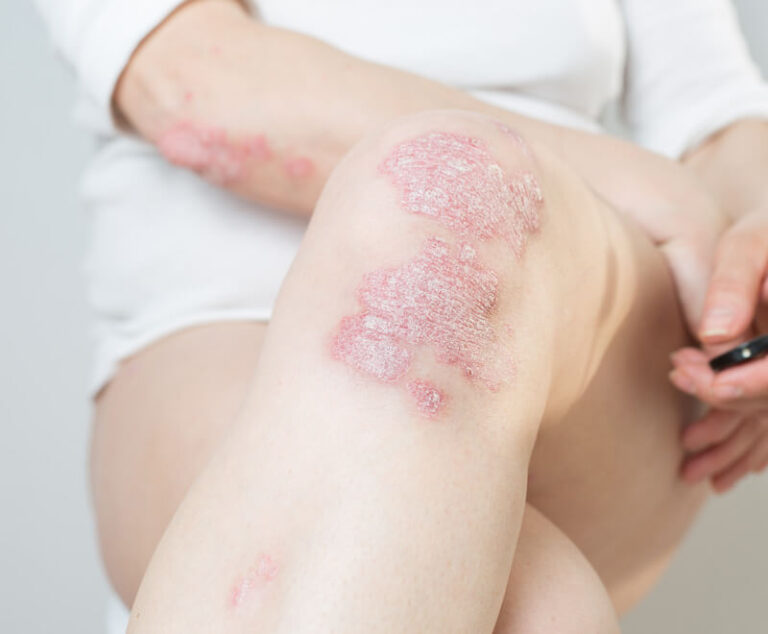
The U.S. Food and Drug Administration (FDA) has approved Wezlana (ustekinumab-auub) as a biosimilar to and interchangeable with Stelara for adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy; active psoriatic arthritis; moderately to severely active Crohn's disease; and moderately to severely active ulcerative colitis.
The U.S. Food and Drug Administration has approved Samsung Bioepis; Halima (adalimumab-bwwd) for the treatment of several autoimmune disorders, including rheumatoid arthritis, psoriatic arthritis, ulcerative colitis and Crohn's disease.
Amgen’s biosimilar to Johnson & Johnson’s rheumatoid arthritis drug, Remicade, has been approved by the U.S. Food and Drug Administration.
The U.S. Food and Drug Administration (FDA) has approved Ziextenzo (pegfilgrastim-bmez), the 24th biosimilar approval in the U.S.