Industry Insight
Information, Observation & Analysis
Therapeutics Articles
With almost a quarter of women receiving inadequate prenatal care in the U.S., more needs to be done to inform women about the myths surrounding this essential healthcare service.
Affecting more than 100,000 American children and adults, sickle cell disease is an inherited hemoglobinopathy that results when a single-nucleotide mutation in the ß-globin gene yields an abnormal “sickle” hemoglobin (HbS). Now, these patients may be eligible for a one-time gene therapy that offers the potential of a durable functional cure by eliminating severe VOCs and associated hospitalizations.
Touted as a more natural approach to hormone replacement therapy, this plant-based anti-aging remedy has evolved from an unregulated safety concern to a mainstay of modern medicine.
Menopause is a normal, natural event in every woman’s life, but it can cause a variety of uncomfortable symptoms. New therapies show promise for bringing relief and an improved quality of life.
For patients with genetic disease, recent evolution of and FDA approval for gene therapies are transforming care and turning an ambitious dream into a life-changing reality. But getting affordable treatments to patients safely and efficiently remains a challenge.
The future of healthcare is being driven by digital transformations and emerging technology that provide preventive, personalized and predictive medicine.
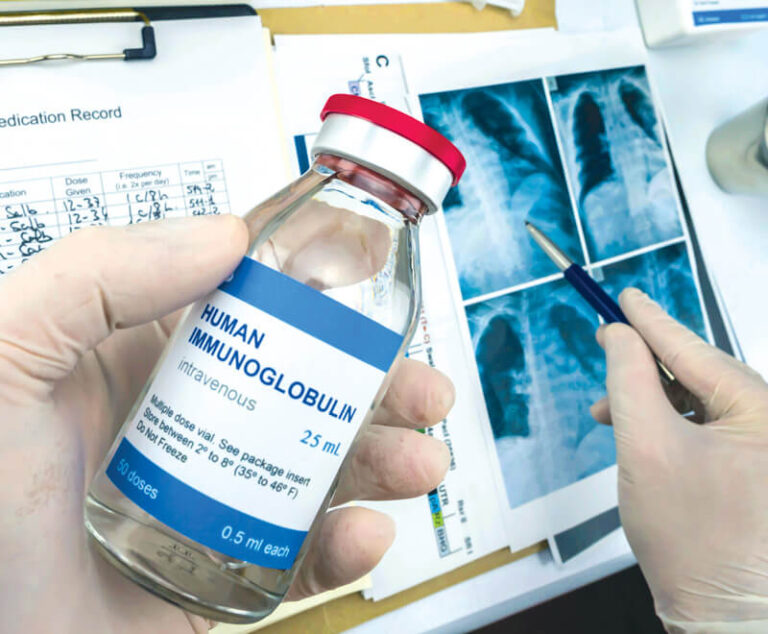
Immune globulin replacement and prophylactic antibiotics are commonly used to prevent infections in patients with secondary hypogammaglobulinemia due to hematological malignancies but have never been directly compared.
While neutropenia can be a life-threatening condition, physicians have many tools to treat it.
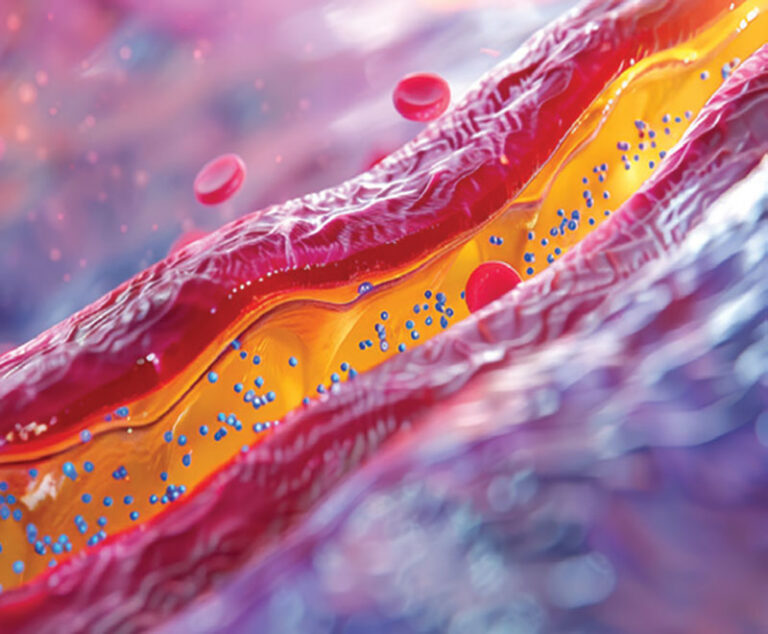
A new vaccine currently in development can effectively and affordably lower levels of “bad’ cholesterol in the body, a health problem that affects almost two in five adults in the U.S.
The U.S. Food and Drug Administration has approved two treatments, Casgevy and Lyfgenia, representing the first cell-based gene therapies for the treatment of sickle cell disease in patients 12 years and older.
CSL Behring is discontinuing all sizes of Hizentra vials in the U.S. by the end of September 2024.
Numerous studies show the efficacy of both IVIG and SCIG for treating CIDP, making these the best treatment options for this rare neurological condition.