New Guidance Recommends All Federal Facilities Have Access to Naloxone
The U.S. Department of Health and Human Services and the General Services Administration have announced new guidance recommending that all federal facilities across the nation include overdose reversal medications in their safety stations on site.
FDA Expands Approval to wilate for Prophylaxis in All Types of VWDr
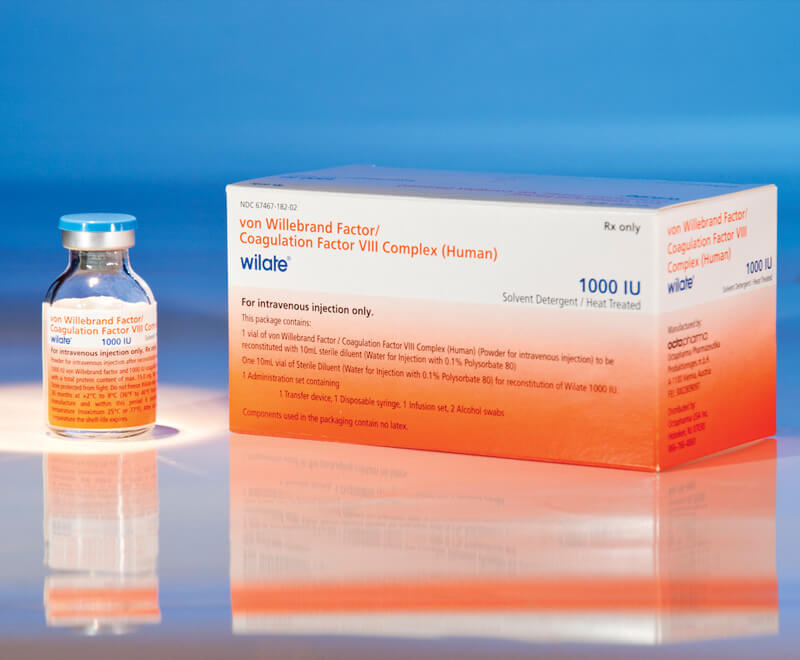
Octapharma USA’s wilate, von Willebrand factor (VWF)/coagulation factor VIII complex (human) lyophilized power for solution for intravenous injection, has been given expanded approval by the U.S. Food and Drug Administration (FDA) for routine prophylaxis to reduce the frequency of bleeding episodes in adults and children aged 6 and older with any type of von Willebrand disease (VWD).
Hyaluronidase-Facilitated Subcutaneous Immune Globulin Effective as Maintenance Therapy for CIDP: Pivotal Trial Results
In a study, researchers found that fSCIG 10% was more effective in preventing CIDP relapse than placebo, supporting its potential use as maintenance CIDP treatment.
Outpatient Albumin Infusions Reduce Hospitalizations in Decompensated Cirrhosis: Retrospective Cohort Study

A team of Australian investigators conducted a retrospective cohort study to determine whether there is a significant benefit in more severely affected cases regularly infused with albumin.
Cancer Drug Shows Promising Results in Clinical Trial

An international early-phase clinical trial has found a “two-for-one” cancer immunotherapy, tebotelimab, is potentially more effective and at least as safe as standard immunotherapies.
Study Shows Positive Results for mRNA-Based Combination Influenza and COVID-19 Vaccine

A Phase I/II study evaluating the safety, tolerability and immunogenicity of Pfizer and BioNTech’s mRNA-based combination vaccine candidates for influenza and COVID-19 among healthy adults 18 to 64 years of age show positive topline results.
FDA Approves Vaccine for Meningococcal Disease in Adolescents
Pfizer’s PENBRAYA, a vaccine for meningococcal groups A, B, C, W and Y, has been approved by the U.S. Food and Drug Administration (FDA).
FDA Approves Celltrion’s Zymfentra to Treat Ulcerative Colitis and Crohn’s Disease
The U.S. Food and Drug Administration (FDA) has approved Celltrion’s Zymfentra, a subcutaneous injection formulation of its infliximab Remsina, for maintenance therapy in adults with moderately to severely active ulcerative colitis and Crohn’s disease following treatment with an infliximab administered intravenously.
FDA Approves Wezlana, First Interchangeable Biosimilar to Stelara
The U.S. Food and Drug Administration (FDA) has approved Wezlana (ustekinumab-auub) as a biosimilar to and interchangeable with Stelara for adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy; active psoriatic arthritis; moderately to severely active Crohn’s disease; and moderately to severely active ulcerative colitis.
HHS Awards $45 Million in Grants to Expand Access to Care for People with Long COVID

The U.S. Department of Health and Human Services announced nine grant awards $1 million each for up to five years to support existing multidisciplinary Long COVID clinics across the country.
