FDA Approves New Safety Labeling for Opioid Medications

FDA approved and implemented updated warning labels for all opioid prescriptions.
FDA Approves First Cell Therapy to Treat Melanoma
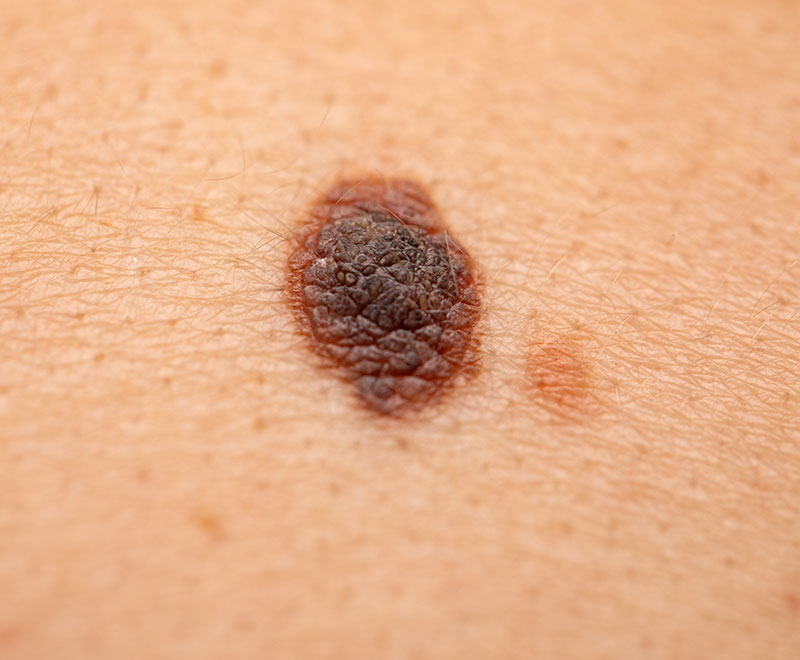
FDA approved Amtagvi, the first cellular therapy indicated for the treatment of adult patients with unresectable melanoma or metastatic melanoma that previously has been treated with other therapies.
Third Humira Biosimilar Is Approved by FDA

FDA has approved Alvotech’s Simlandi (adalimumab-ryvk) as the third interchangeable Humira biosimilar.
New Guidance Recommends All Federal Facilities Have Access to Naloxone
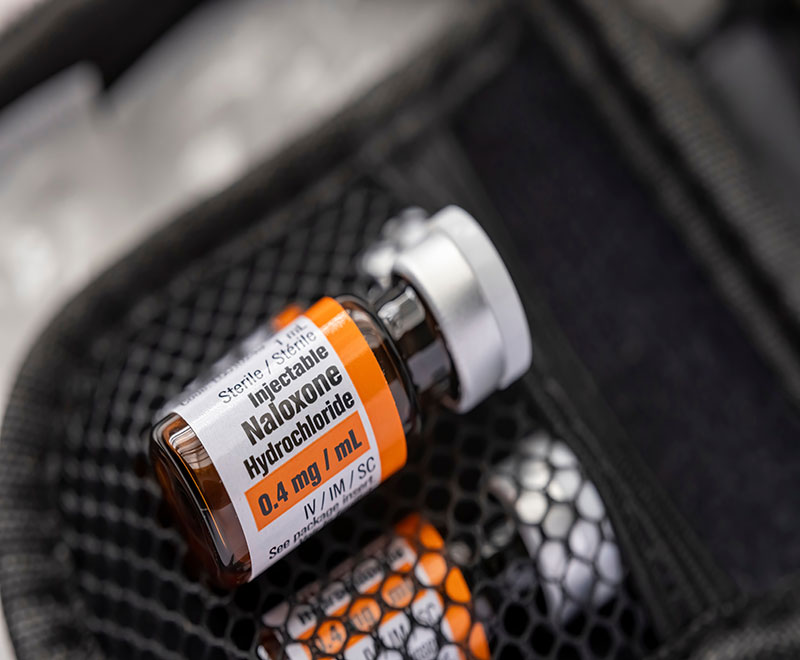
The U.S. Department of Health and Human Services and the General Services Administration have announced new guidance recommending that all federal facilities across the nation include overdose reversal medications in their safety stations on site.
FDA Approves Antibody to Protect Infants Against RSV
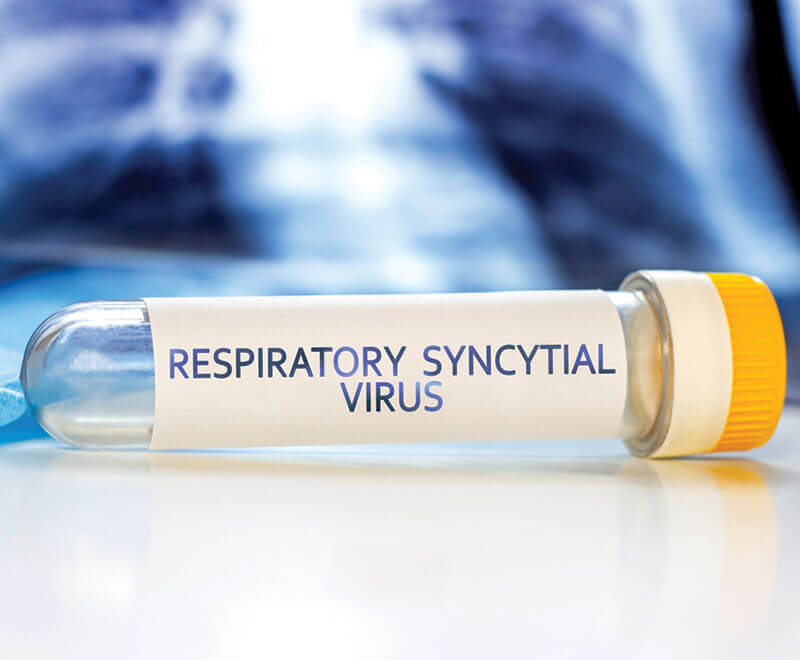
The U.S. Food and Drug Administration (FDA) has approved nirsevimab to protect newborns from respiratory syncytial virus (RSV).
FDA Approves First Postpartum Depression Oral Treatment

The U.S. Food and Drug Administration has approved Zurzuvae (zuranolone), the first oral medication indicated to treat postpartum depression (PPD) in adults.
First Over-the-Counter Contraceptive Pill Gets FDA Approval
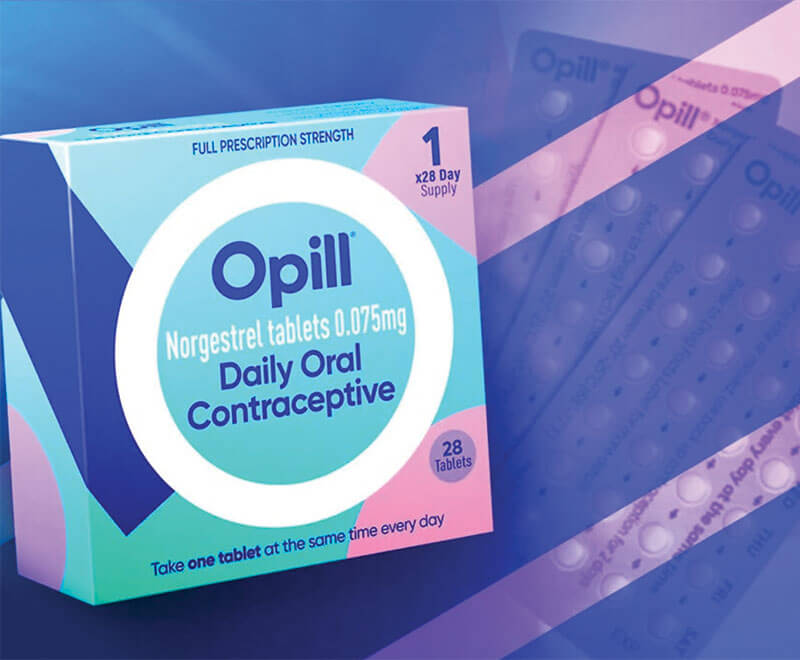
The U.S. Food and Drug Administration has approved the birth control pill Opill (norgestrel), manufactured by Perrigo, to be available over-the-counter — the first nonprescription birth control pill in the United States.
FDA Approves Pfizer’s ABRYSVO Vaccine for the Prevention of RSV

Pfizer’s ABRYSVO, a respiratory syncytial virus (RSV) vaccine, has been approved by the U.S. Food and Drug Administration for the prevention of lower respiratory tract disease (LRTD) and severe LRTD caused by RSV in infants from birth up to 6 months of age by active immunization of pregnant women at 32 through 36 weeks gestational age.
First Once-Daily Oral Plaque Psoriasis Drug Approved by FDA
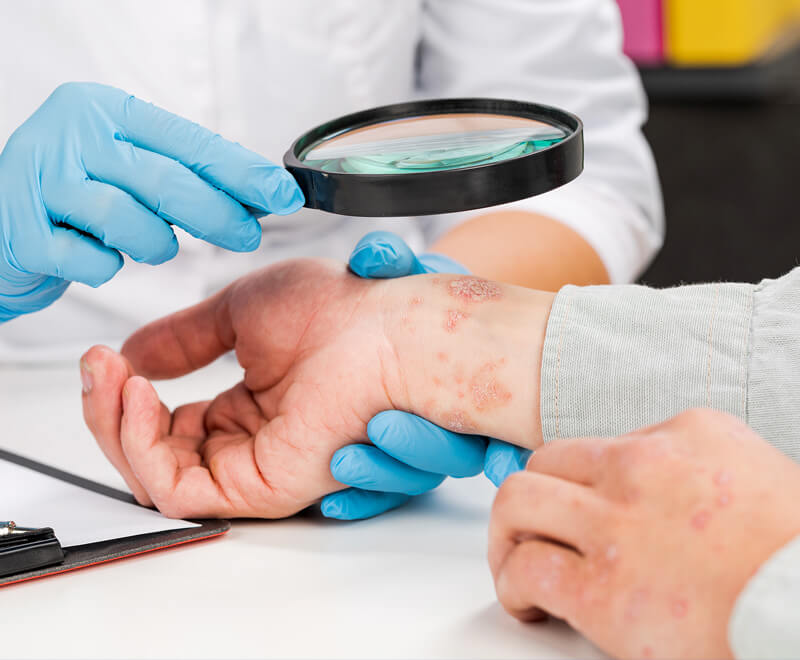
The U.S. Food and Drug Administration has approved Sotyktu (deucravacitinib), a once-daily oral pill by Bristol Myers Squibb, for adults who have plaque psoriasis (a chronic, systemic, immune-mediated disease) severe enough to make them candidates for systemic therapy and phototherapy.
FDA Approves Humira Biosimilar to Treat Autoimmune Disorders
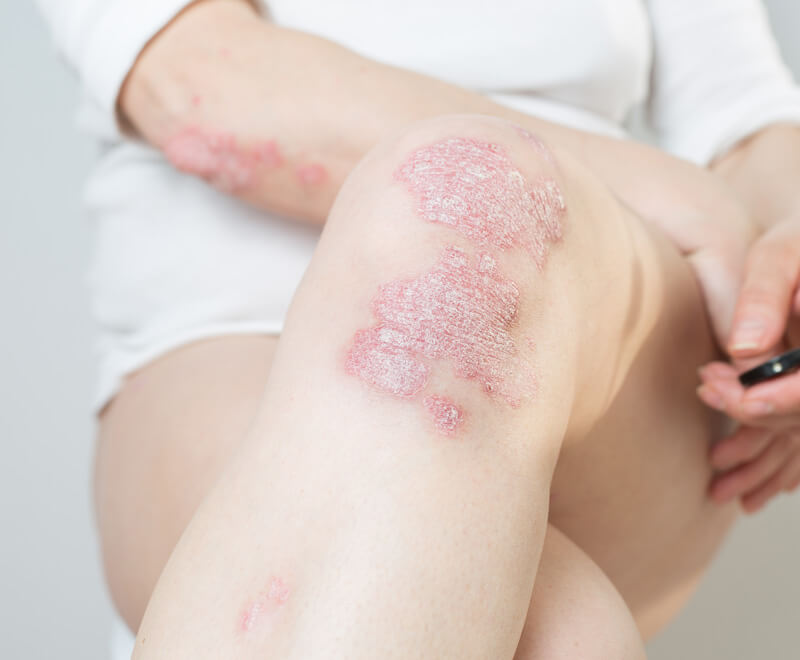
The U.S. Food and Drug Administration has approved Samsung Bioepis; Halima (adalimumab-bwwd) for the treatment of several autoimmune disorders, including rheumatoid arthritis, psoriatic arthritis, ulcerative colitis and Crohn’s disease.
